Guide to categorising your chemical importation and manufacture
All industrial chemical importers and manufacturers must categorise their chemical introduction. This step-by-step guide takes you through the process of categorisation.
| Version | Description | Date |
| 3.4 | Minor edits to 'Appendix - acceptable test guidelines for categorisation' and 'Human health hazard band B hazard characteristics' pages plus 118 chemicals added to the list of chemicals with high hazards for categorisation. | 1 September 2025 |
| 3.3 | Step 0 - introductions that are in the listed category Minor updates and links added to improved guidance on working out if you need to submit a specific information requirement form and links to newly published guidance on submitting an SIR form in AICIS Business Services. | 4 August 2025 |
| 3.2 | Refined requirements to prove the absence of bioaccumulation potential in step 5.4 and specific target organ toxicity after repeated exposure in step 4.4 | 24 September 2024 |
| 3.1 | All references and requirements related to 'personal vaporiser' removed. The use of chemicals in vaping goods, or as vaping goods, are not an industrial use from 1 July. | 1 July 2024 |
| 3 | Changes incorporated into the Categorisation Guide to reflect amendments to the Categorisation Guidelines and amendments to the Industrial Chemicals (General) Rules 2019 both of which came into effect on 24 April 2024. | 24 April 2024 |
| 2.4 | Step 2 - Introductions that are categorised as exempted Examples added to the section dealing with polymers that are comparable to listed polymers and information added with examples regarding determining percent by weight. | 24 October 2023 |
| 2.3 | Step 4.1 and Step 5.1 The draft OECD TG 125 on particle size and particle size distribution on nanomaterials is now final and part of our guidance. We've updated the content related to nanoscale introductions as a result. Step 6 Clearer instructions on how to use the health and environment risk to work out the introduction category. | 30 May 2023 |
| 2.2 | Step 3 - Introductions that are in the reported category Provided more clarity around 3.2 Low-risk flavour and fragrance blend introductions regarding human health hazard characteristics and environmental hazard characteristics requirements. | 17 February 2023 |
| 2.1 | Step 3 - Introductions that are in the reported category Added the following 2 chemicals to question 2, part 3.1 Introductions of 10 kg or less in an AICIS registration year. Benzene, 1,2,3,4,5-pentachloro- Benzene, hexachloro- | 8 February 2023 |
| 2.0 | Step 0 - Introductions that are in the listed category Improvements made to the page around next steps if you have categorised your introduction as listed by adding links to a new page in the guide 'Your obligations after categorisation'. Step 2 - Introductions that are in the exempted category Improvements made to the page around next steps if you have categorised your introduction as exempted by adding links to a new page in the guide 'Your obligations after categorisation'. Step 3 - Introductions that are in the reported category Criteria added for a new type of low-volume reported introduction of 10 kg or less per year. We've also provided more clarity around next steps depending on the outcome at step 3. This includes adding links to a new page 'Your obligations after categorisation' if an introducer has categorised their introduction as reported at this step. Step 6 - Complete your categorisation We've moved some content on this page on reporting and record-keeping obligations to the new page 'Your obligations after categorisation'. Your obligations after categorisation This new page gives an overview about reporting and record-keeping obligations for introducers after they've categorised their introduction as one of the following:
| 25 November 2022 |
| 1.4 | Steps 4.1 and 5.1 – Is your chemical a certain chemical at the nanoscale? More options added in a question and answer format to help introducers work out if they are introducing this type of chemical. Steps 4.1 and 5.1 – Where an introduction is a specified class of introduction More information added, including when introductions are specified classes, our concerns about them, and that extra information will be required from introducers when submitting an assessment application for a specified class of introduction. Content has been added under these headings:
Steps 4.5 and 5.5 – Special cases - introductions that cannot have a very low indicative human health risk and introductions that cannot have a very low indicative environment risk Added this extra point to make it clear this type of chemical cannot have a very low indicative risk for human health or the environment: 'OR
Step 6 – Next steps: If your introduction is categorised as assessed Added the next step required if the chemical is on the Inventory. Clarified the outcome when the chemical is not on the Inventory. | 30 March 2022 |
| 1.3 | Step 1: added words shown in bold text: '...can not be categorised as an exempted or reported introduction unless it is both of the following:
Step 2: clearer explanation of the nanoscale criteria for research and development and chemicals resulting from non-functionalised surface treatment of listed chemicals; removed 'Tylosin, (2R,3R)-2,3-dihydroxybutanedioate (1:1)' with CAS number 74610-55-2 from the comparable chemicals table and improved the instructions on how to use the table. Step 3: clearer explanation of the nanoscale criteria for research and development. Step 5.3: added statement to clarify that a chemical with an end use in an air freshener is not a 'designated kind or release into the environment' Correction: Environment hazard band A hazard characteristics. indented the following paragraphs to correctly align as follows:
| 23 November 2021 |
| 1.2 |
| 28 May 2021 |
| 1.1 | Replaced references to Categorisation Guidelines in step 4.4 and 5.4 with the details (from the Guidelines) about hazard bands; improvements and more information in 'Before you start categorising your introduction'. Added appendices: acceptable test guidelines for categorisation and in silico predictions for categorisation. | 22 December 2020 |
| 1 | Original | 1 July 2020 |
Drawing on information in the IC Act, General Rules and the Industrial Chemicals Categorisation Guidelines, this step-by-step guide with supporting self-guided decision tools helps you categorise your chemical importation or manufacturer as listed or exempted or reported or assessed.
Introducer means a person or business that manufacturers an industrial chemical in Australia or imports an industrial chemical into Australia.
Introduction means the importation or manufacture of an industrial chemical in Australia by an introducer. Each introduction of a chemical is unique because it includes how the chemical is used, who uses or accesses the chemical, concentration and other factors. Learn more about introduction.
In this guide
Before you start categorising your introduction
Who is this guide for?
Anyone who plans to manufacture or import industrial chemicals (or products that contain industrial chemicals) into Australia for commercial, research or any business-related purposes.
This guide is designed to help you work out which AICIS introduction category applies to each of your chemical introductions.
Note: you don't need to use this guide or categorise your chemical introductions if it's only for personal or hobby use, or your introduction is a type that doesn't need to be categorised under our laws.
AICIS introduction categories
Listed introductions
A chemical introduction that is categorised as ‘listed’ means it is on our Inventory and already available for industrial use in Australia. Your business must be registered with us and you must meet any terms of the listing for that chemical. You must keep records and submit an annual declaration at the end of each registration year. There's no fee for listed introductions.
Exempted introductions
A chemical introduction that is categorised as ‘exempted’ means it meets a very strict set of criteria that is considered very low risk to both human health and the environment. If your introduction meets the criteria and you're already registered with us, you can introduce the chemical without telling us beforehand. You must keep records and submit an annual declaration at the end of each registration year. Some exempted introductions also require you to submit a once-off post-introduction declaration the first time that you introduce the chemical. There's no fee for exempted introductions.
Reported introductions
A chemical introduction that is categorised as ‘reported’ means it meets our criteria to be considered low risk to human health or the environment. You must be registered with us and submit a once-off pre-introduction report before you start introducing it. You must keep records and submit an annual declaration at the end of each registration year. There's no fee for reported introductions and no fee to submit a pre-introduction report.
Assessed introductions
A chemical introduction that is categorised as ‘assessed’ means we consider it to be medium to high risk to human health or the environment. It cannot be in the exempted or reported categories. If the chemical is not on the Inventory, then we must assess your introduction and issue an assessment certificate before you can import or manufacture it. The chemical will be listed on the Inventory 5 years after we issue a certificate.
You must keep records and submit an annual declaration at the end of each registration year. There is a fee to apply for an assessment certificate.
Commercial evaluation
If you meet our commercial evaluation criteria, you can apply for a commercial evaluation authorisation as an alternative option to the exempted, reported or assessed category. The chemical will not be listed on the Inventory. You must keep records and submit an annual declaration at the end of each registration year. There is a fee to apply for a commercial evaluation authorisation.
The Minister can also authorise introductions under exceptional circumstances.
An overview of AICIS introduction categories. All introducers must categorise each chemical introduction into one of these categories.
Step 0: Introductions that are in the listed category
- If you are selling soap, read our soaps guide to learn more.
What is a listed introduction?
A chemical importation or manufacture (introduction) is in the listed category if it is both of the following:
- The chemical is on the Australian Inventory of Industrial Chemicals (Inventory).
- The AICIS-registered person or business (introducer) can import or manufacture the chemical within the terms of Inventory listing described in the chemical’s Inventory record.
Who has the chemical identity information?
To search the Inventory, either you or another person or business (for example, your overseas supplier or manufacturer), must know at least one of the following about your chemical’s identity:
- CAS number (recommended)
- CAS name, or AICIS-Approved Chemical Name (AACN) if AICIS has assessed the chemical.
Since the Inventory is based on CAS names and numbers, searching the Inventory using INCI names, product names or common names isn’t recommended. See more search tips, including how you might find CAS numbers or names.
Choose your scenario to learn what to do next.
Scenario 1: Introducer knows the chemical’s CAS number, CAS name or AACN.
Introducer can complete the questionnaire - go to Q1.
Scenario 2: Introducer does not know the CAS number, CAS name or AACN – this is proprietary information and cannot be given to the introducer.
Ask your chemical identity holder to help categorise your chemical introduction. Provide a link to this page and ask them to work through the questionnaire on your behalf.
Ideally, the chemical identity holder should give you the answer to each question, but if this is not possible, then ask for a summary that confirms:
- whether the chemical is on the Inventory
- if the chemical is on the Inventory, whether the listing contains:
- a defined scope of assessment
- conditions of introduction of use
- a specific information requirement
- an obligation relating to the Rotterdam Convention or Minamata Convention on Mercury and requiring approval.
You must help the introducer to categorise their introduction. Complete the questionnaire and either give the introducer the answer to each question (preferred), or provide the introducer with a summary that confirms:
- whether the chemical is on the Inventory
- if the chemical is on the Inventory, whether the listing contains:
- a defined scope of assessment
- conditions of introduction of use
- a specific information requirement
- an obligation relating to the Rotterdam Convention or Minamata Convention on Mercury and requiring approval.
Scenario 3: The person or business who knows the chemical identity information has told you that the chemical does not have an assigned CAS number or CAS name.
This means the chemical is unlikely to be on the Inventory and probably won’t be categorised as a listed introduction.
If this applies, continue with the categorisation process by going directly to Step 1: Introductions that cannot be exempted or reported.
Questionnaire to work out if an introduction is in the listed category
1. Is the chemical on the Inventory?
Search the Inventory
See search tips below.
If the answer is yes, go to question 2.
If the answer is no, your introduction is not eligible for the listed category. Continue with this guide to work out if your introduction is in the exempted, reported or assessed category – go directly to Step 1: Introductions that cannot be exempted or reported.
2. Does the chemical record show any of these?
- Defined scope of assessment
- Conditions of introduction or use
- Specific information requirement
- More information relating to Rotterdam Convention or Minamata Convention on Mercury
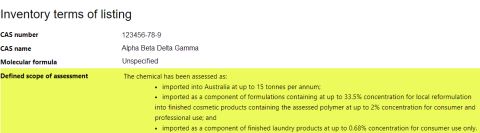
A defined scope of assessment describes the parameters of any previous assessment of a chemical, such as how the chemical is used, the introduction volume or quantity, and its concentration in products.
If there is a ‘defined scope of assessment’, it will display under the heading ‘Inventory terms of listing’. See this example:
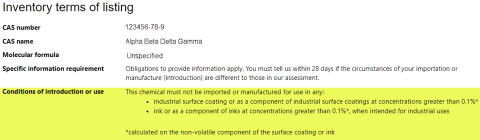
Conditions of introduction or use are restrictions we've imposed on the importation or manufacture of a chemical. For example, how much (volume) you can import or manufacture (introduce) and where the chemical is permitted to be introduced or used. If there is a condition, it will be listed under the heading ‘Inventory terms of listing’. See this example:
Chemicals subject to the Rotterdam Convention or Minamata Convention on Mercury. If your chemical is subject to one of these conventions, it will be stated under ‘More information’. See this example:
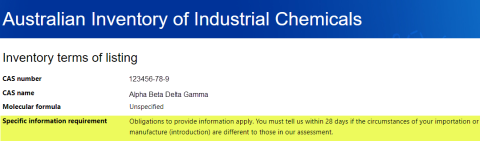
A specific information requirement is an obligation for introducers (or someone on the introducers’ behalf) to provide information about a chemical introduction under certain situations. An example is below:
Select a, b, or c:
a. No, it has none of these.
b. Yes, it contains a defined scope of assessment, or conditions of introduction or use or obligations under a Convention.
c. Yes, but it ONLY has a specific information requirement.
If you answered a, the chemical introduction is in the ‘listed’ category. That is, the importation or manufacture of the chemical is categorised as a listed introduction. Next: Your obligations after categorisation for listed introductions.
If you answered b, go to question 3.
If you answered c, go to question 9.
3. Does the chemical record indicate that prior authorisation is required before introducing or exporting, because the chemical is either subject to the Rotterdam Convention or the Minamata Convention on mercury?
If the answer is no, go to question 4.
If the answer is yes, and there are no other terms of listing (‘conditions of introduction or use’, ‘defined scope of assessment’ or ‘specific information requirements’), then the introduction is in the listed category. But you need to apply for authorisation to introduce or export your chemical:
- Rotterdam Convention: Apply for authorisation to import or export
- Minamata Convention on Mercury: Apply for authorisation to import or export
If you receive authorisation, you must comply with AICIS obligations for listed introductions (if applicable) as well as any others that apply under the Convention.
If the answer is yes, and there is at least one other term of listing (‘conditions of introduction or use’, ‘defined scope of assessment’ or ‘specific information requirements’), contact us using our online form and select the topic ‘Categorising chemical introductions’.
4. Does the chemical record show any ‘conditions of introduction or use’?
If the answer is yes, go to question 5.
If the answer is no, go to question 6.
5. Will the chemical be introduced within the boundaries of the conditions of introduction or use?
Read the conditions of introduction or use on the chemical’s Inventory record and compare this with the way you’re importing/manufacturing and using the chemical.
Conditions of introduction or use are restrictions we've imposed on the importation or manufacture of a chemical. The conditions are included to manage risks to human health or the environment (if required). For example, conditions on the maximum quantity or volume of chemical that can be imported or manufactured and where the chemical is permitted to be used.
Example
Conditions of introduction or use: the chemical can only be used at a specific site ‘A’.
You plan to use the chemical at other sites.
Outcome: your introduction does not meet the condition. Your answer to this question is ‘no’.
If the answer is yes, go to question 6.
If the answer is no, and there are no other terms of listing (‘defined scope of assessment’ or ‘specific information requirements’), then you must not manufacture or import a chemical in circumstances outside of the conditions. You can apply to vary the conditions of introduction or use. If we approve it, your introduction can be in the listed category. Next: Apply to vary the Inventory terms of listing (fee applies).
If the answer is no and there are other terms of listing (‘defined scope of assessment’ or ‘specific information requirements’), contact us using our online form and select the topic ‘Categorising chemical introductions’.
6. Does the chemical record show a ‘defined scope of assessment’?
If the answer is yes, go to question 7.
If the answer is no, go to question 8.
7. Is the introduction within the parameters of the defined scope of assessment?
If the answer is yes, go to question 8.
If the answer is no, you have 3 options:
Option 1: Continue with this guide to work out if your introduction can be in the exempted or reported category. If categorised as exempted or reported, you must comply with all associated regulatory obligations. Go to Step 1: Introductions that cannot be exempted or reported.
Option 2: You can apply to change the parameters of the defined scope of assessment for a chemical's listing to match your proposed introduction circumstances. If we approve it, your introduction can be in the listed category. Next: Apply to vary the Inventory terms of listing (fee applies). Note – if the chemical record also shows a ‘specific information requirement’, contact us using our online form and select the topic ‘Categorising chemical introductions’.
Option 3: If the chemical is being introduced to test its commercial potential in Australia, the introducer can apply for a commercial evaluation authorisation – fee, time limit and strict eligibility criteria apply.
8. Does the chemical record show a ‘specific information requirement’?
If the answer is yes, go to question 9.
If the answer is no, the chemical introduction is in the ‘listed’ category. That is, the importation or manufacture of the chemical is categorised as a listed introduction.
Next: ‘Your obligations after categorisation for listed introductions’.
9. Do you need to submit information to AICIS under the ‘specific information requirement’?
To answer this question, you need to use our guidance to work out if you need to submit information based on your chemical’s use and other aspects of your introduction.
A specific information requirement does not automatically mean you need to submit the specific information requirement (SIR) form.
If the answer is no, because you determined that the chemical introduction is within the terms of listing, then you do not need to provide information about your chemical introduction. The importation or manufacture of your chemical is categorised as a listed introduction.
Next: ‘Your obligations after categorisation for listed introductions’.
If the answer is yes after working through our guide, you must submit an SIR form in AICIS Business Services) within the specified timeframe.
Before you submit, we recommend reading our guidance to help you correctly complete the SIR form that applies to your circumstance. Our guides include tips and a preview of the questions in the form.
By doing this, you have determined that your chemical introduction will be in accordance with the terms of listing. Therefore, the importation or manufacture of your chemical is categorised as a listed introduction.
Next: ‘Your obligations after categorisation for listed introductions’.
Tips on how to search the Inventory
The Australian Inventory of Industrial Chemicals (Inventory) is an online database of industrial chemicals that are being manufactured or imported into Australia.
If you have a mixture or product that contains more than one chemical, you must search for each one separately.
Search the Inventory using the chemical's CAS number. If you don't know the CAS number or the chemical does not have an assigned CAS number, search the Inventory using the chemical's CAS name (using the keyword search).
No search results or too many results
Did you search using the chemical's CAS number or CAS name?
If no, then we recommend that you search using the chemical's CAS number (preferred), or CAS name. This is because a term of Inventory listing is the CAS name and CAS number for a chemical.
If yes, then check if it is one of these reasons:
- The chemical meets our legal definition of a naturally occurring chemical - chemicals that meet this definition do not need to be on the Inventory.
- It’s a mixture (such as an alloy or hydrate) – the Inventory only contains names of chemicals, not mixtures.
- You entered an incorrect CAS number or it doesn’t match the CAS number format. For example you may have added a space between the numbers or hyphens.
- You entered an outdated CAS number. Sometimes CAS replaces a chemical’s CAS number with a new one, so you need to make sure that you’re using the updated CAS number. You can check if you have an up-to-date CAS number for your chemical by searching chemical databases such as ChemIDPlus and SciFinder-n.
Still no result after searching the chemical's CAS number?
You can ask us to check if your chemical is confidentially listed on the Inventory. This is because there are some chemicals that are listed on the Inventory where the CAS name and CAS number are protected as confidential business information (CBI). If it is not confidentially listed on the Inventory, then you must proceed to Step 1 of the Categorisation Guide to work out your chemical introduction category.
Trade names, product names and INCI names
The Inventory is a database of chemicals, not products, mixtures or formulations. Therefore it does not contain product names, trade or marketing names and rarely contains INCI or common chemical names. We recommend finding a CAS number or CAS name for each chemical that you want to search. For example:
Search using the CAS number or name |
Don’t search using trade or common names |
| 107-21-1 / 1,2-Ethanediol 57-55-6 / 1,2-Propanediol | Antifreeze |
| 144-55-8 / Carbonic acid, monosodium salt | Baking soda |
| 77-92-9 / 1,2,3-Propanetricarboxylic acid, 2-hydroxy- | Citric acid |
| 9005-25-8 / Starch | Corn starch |
| 7487-88-9 / Sulfuric acid magnesium salt (1:1) | Epsom salt |
| 56-81-5 / 1,2,3-Propanetriol | Glycerine |
| 8000-28-0 / Essential oils, lavender | Lavender oil |
| 13463-67-7 / Titanium oxide (TiO2) | Liquid paper |
| 1310-73-2 / Sodium hydroxide (Na(OH)) | Lye |
| 68917-75-9 / Oils, wintergreen | Wintergreen oil |
Important: These CAS numbers are examples only. It is the introducer’s responsibility to correctly identify and know the chemistry of their introductions.
Watch our video: Tips on searching for chemicals on the Inventory
Step 1: Introductions that cannot be exempted or reported
Instructions
The introductions described on this page are not eligible for the exempted or reported categories. Start at 1 and work down the page to see if any of these apply to your introduction.
1. Chemical is on the Inventory but you don't meet the conditions of introduction or use
If your chemical is on the Inventory with a condition of introduction or use, you must ensure you can meet the conditions when you introduce the chemical. An Inventory listing can include a condition about:
- the total annual volume of the chemical that you can introduce
- the location where you can introduce or use the chemical
If your introduction is on the Inventory with a condition of introduction or use and you cannot meet the conditions, it is not authorised under our exempted or reported categories. You must apply to vary the terms of the Inventory listing. We must approve your application before you can start introducing the chemical.
If this applies to your introduction, go to Apply to vary the terms of a listing on the Inventory (fee applies).
If this does not apply to your introduction, continue to 2. Persistent organic pollutants (POPs) and chemicals subject to the Rotterdam Convention.
If this applies to your introduction, go to Apply to vary the terms of a listing on the Inventory (fee applies).
If this does not apply to your introduction, continue to 2. Persistent organic pollutants (POPs) and chemicals subject to the Rotterdam Convention.
Some introductions are not eligible for the exempted or reported categories.
2. Persistent organic pollutants (POPs) and chemicals subject to the Rotterdam Convention
Your introduction is in the assessed category and you must apply for an assessment certificate if any of these scenarios apply, unless you meet an exception*:
*Exception 1: your introduction is in the listed category.
*Exception 2: chemical is only used for research or analysis and introduced at 100 kg or less in an AICIS registration year.
The Persistent Organic Pollutants Review Committee has decided that the chemical meets the POPs screening criteria set out in Annex D of the Stockholm Convention. These chemicals are shown below. For more information about these chemicals, refer to POPRC Recommendations.
Chemical name CAS number Chlorypyrifos 2921-88-2 Chlorinated paraffins with carbon chain lengths in the range C14-17 and chlorination levels at or exceeding 45 per cent chlorine by weight - Long-chain perfluorocarboxylic acids, their salts and related compounds Various Octabromodiphenyl ether 32536-52-0
- The AICIS Executive Director has decided that the chemical meets the Annex D screening criteria for POPs while making the decision about issuing an assessment certificate for that chemical, or based on an AICIS evaluation done on that chemical.
This applies to this chemical:
Chemical name: Benzene, 1,1'-(1,2-ethanediyl)bis[2,3,4,5,6-pentabromo- (also known as decabromodiphenylethane or DBDPE)
CAS number: 84852-53-9
If you wish to trade (import or export) a chemical that is listed in Annex III to the Rotterdam Convention and in Section 71 or 73 of the Industrial Chemicals (General) Rules, you must apply in writing and pay a fee. This application process is known as the prior informed consent (PIC) procedure.
Next: Step 2: Introductions that are categorised as exempted
Step 2: Introductions that are categorised as exempted
Do you meet criteria on this page?
2.1 Australian-made soap using lye and maximum of 100 kg of fat/oil a year
For the purpose of this guide, ‘soap-chemical’ refers to the chemical that is made by mixing lye (sodium hydroxide or potassium hydroxide) with an oil or fat.
2.1.1 Is your soap-chemical made (manufactured) in Australia?
If yes, go to 2.1.2.
If no, go to 2.2 or skip to Step 3 if your introduction is not covered elsewhere on this page.
2.1.2 Is your soap-chemical made using a saponification process with a fat or oil and lye (either aqueous sodium hydroxide or aqueous potassium hydroxide)?
If yes, go to 2.1.3.
If no, go to 2.2 or skip to Step 3 if your introduction is not covered elsewhere on this page.
2.1.3 Is the total volume of the fat or oil used to make the soap-chemical no more than 100 kg in an AICIS registration year (1 September to 31 August)?
If yes, then your introduction is in the exempted category for introduction of manufactured soap. Skip to Your obligations after categorisation.
If no, go to 2.2 or skip to Step 3 if your introduction is not covered elsewhere on this page.
Example 1
Alita makes soap in Australia using aqueous sodium hydroxide and 100 kg of coconut oil per AICIS registration year (1 September to 31 August).
Introduction of Alita's soap-chemical can be categorised as exempted as all criteria are met.
Example 2
Darren makes soap in Australia using aqueous sodium hydroxide, 50 kg of cottonseed oil and 80 kg of linseed oil per AICIS registration year (1 September to 31 August).
Introductions of Darren's soap-chemicals can be categorised as exempted as all criteria are met.
2.2 Chemicals that are imported and subsequently exported
Your introduction is categorised as exempted if all of the following apply:
- the entire volume is imported and subsequently exported out of Australia
- the packaging in which your chemical is immediately contained is never opened
- whilst your chemical is in Australia, it remains under the control of either customs (for longer than 25 working days) or the introducer.
Note: if your chemical is under customs controls whilst in Australia and leaves Australia within 25 days, then your introduction is an excluded introduction.
2.3 Chemicals that are only used for research and development
Your introduction is categorised as exempted if all of the following apply (note that the volume of chemical that you can introduce in a registration year is lower, unless you can demonstrate that the nanoscale criteria do not apply to your introduction):
- you only use your chemical for research and development, or you make it available to another person who only uses it for research and development
- you don’t make your chemical available to the public on its own, in combination with other industrial chemicals or as part of an article
- you use control measures to eliminate or minimise any risks to the environment and any risks to the people involved in using the chemical for research and development
and point 1 or 2 or 3 applies:
- You will introduce up to 250 kg of your chemical in a registration year and you can demonstrate that all quantities of your chemical are not introduced as a solid or in a dispersion. To prove that your chemical is not introduced as a solid or in a dispersion, you might have an SDS or product information sheet that indicates the appearance (for example, in liquid form).
- You will introduce up to 250 kg of your chemical in a registration year and you can demonstrate that all quantities of your chemical do not consist of solid particles in an unbound state or as an aggregate or agglomerate, where at least 50% (by number size distribution) of the particles have at least one external dimension in the particle size range of 1 to 100 nm. To prove that your chemical does not consist of solid particles in an unbound state or as an aggregate or agglomerate, where at least 50% (by number size distribution) of the particles have at least one external dimension in the nanoscale, you might have a study report about the particle size distribution of your chemical.
- You will introduce up to 10 kg of your chemical in a registration year.
If you meet the research and development criteria and your chemical is at the nanoscale, or you had not determined at the time of introduction that it is not at the nanoscale, your introduction can only be in the exempted category if you introduce 10kg (or less) in a registration year.
Learn more about categorising chemicals introduced for research and development
2.4 Polymers of low concern (PLC)
Your introduction is categorised as exempted if it meets the criteria for a polymer of low concern and it’s not a high molecular weight polymer that has lung overloading potential.
If you are introducing polymers of low concern, you must submit a once-off exempted introduction declaration by 30 November (following the end of our registration year).
2.5 Low-concern biological polymers
Your introduction is categorised as exempted if it’s a low-concern biological polymer that meets all of the following criteria:
- the chemical is a biological chemical (that is, it’s derived from a living or once-living organism, without further modification, or produced by a living or once-living organism, without further modification)
- the chemical is a polymer
- the polymer meets most of the polymer of low concern criteria, except that it’s not stable, meaning that it substantially degrades, decomposes or depolymerises during use into simpler, smaller weight chemicals
Examples of low-concern biological polymers are keratin and collagen. Enzymes are not polymers because of the lack of variability in molecular weight.
If you are introducing low-concern biological polymers, you must submit a once-off exempted introduction declaration by 30 November (following the end of our registration year).
2.6 Polymers that are comparable to listed polymers
Your introduction is categorised as exempted if ALL of the following apply:
- your chemical is a polymer
- your polymer contains exactly the same reactants (must have each of the reactants) as another polymer that is already listed on the Inventory
- your polymer contains one or more other reactants (the additional reactants) that the listed polymer does not
- each additional reactant is present at no more than 2% by weight of the polymer
You must also comply with any regulatory requirements associated with the listed polymer.
Example 1
Sean plans to introduce a polymer manufactured from reactants A, B and C (Polymer ABC), which is not on the Inventory.
He knows that reactant C is present at no more than 2% by weight of the polymer. He searches the Inventory for the comparable polymer, Polymer AB, and finds that it is listed on the Inventory. This means Sean can introduce Polymer ABC as an exempted introduction if he's registered with us and his introduction meets any regulatory obligations for the listed polymer, Polymer AB.
Later, Sean decides he wants to introduce Polymer ABC with reactant C present at greater than 2% by weight of the polymer. This would mean it is no longer comparable to the listed polymer, Polymer AB. Sean must re-categorise his introduction.
Example 2
Kate plans to introduce a polymer manufactured from reactants W, X, Y and Z (Polymer WXYZ), which is not on the Inventory. She knows that reactants Y and Z are each present at no more than 2% by weight of the polymer.
She searches the Inventory for the comparable polymers (i.e., Polymer WX, Polymer WXY or Polymer WXZ) and finds that Polymer WXY is listed on the Inventory.
This means Kate can introduce Polymer WXYZ as an exempted introduction if she's registered with us and her introduction meets any regulatory obligations for the listed polymer, Polymer WXY.
Later, Kate plans to introduce Polymer WXYZ with reactant Z present at greater than 2% by weight of the polymer. This would mean it is no longer comparable to the listed polymer, Polymer WXY. Kate must re-categorise her introduction.
Determining percent by weight
The ‘percent by weight’ of each reactant may be determined by the percent charged to the reactor, or the percent incorporated in the polymer as established analytically. When using the percent incorporated method, the percent by weight of the reactant is based on the minimum weight of reactant to account for the actual weight of the reactant or fragments of the reactant incorporated in the polymer.
Example 1 – percent incorporated method
Kamal manufactures his polymer using the radical initiator azobis[isobutyronitrile] (AIBN, MW = 164 g/mol), charged into the reactor at 3%. This class of initiator is known to produce radicals that contain the nitrile moiety (CN, MW = 26 g/mol). Kamal analyses the polymer and finds that it contains 0.39% by weight nitrile, which originates only from AIBN. This 0.39 g of nitrile fragment in 100 g of polymer corresponds to 0.015 moles of nitrile fragment (0.39 g / 26 g/mol = 0.015 moles) in 100 g of polymer. As 1 mole of AIBN reactant produces 2 moles of nitrile fragment, the amount of AIBN reactant to account for the actual amount of nitrile fragment incorporated in the polymer is 0.015/2 = 0.0075 moles AIBN in 100 g of polymer, or 1.23 g (0.0075 mol x 164 g/mol) AIBN in 100 g of polymer. This corresponds to a weight percent of AIBN reactant incorporated of 1.23%.
Example 2 – percent incorporated method
Sienna manufactures a polymer containing free carboxylic acid groups which is neutralised using a large excess of sodium hydroxide (NaOH, MW = 40 g/mol). The total amount of base charged to the reactor is 10%. Sienna analyses the resulting polymer salt and find that it contains 1.52% by weight of sodium (atomic weight = 23 g/mol), which originates only from the base. This 1.52 g of sodium in
100 g of polymer corresponds to 0.066 moles (1.52 g / 23 g/mol) of sodium in 100 g of polymer. The amount of NaOH reactant to account for the actual amount of sodium incorporated in the polymer is 0.066 moles NaOH in 100 g of polymer, or 2.64 g (0.066 mol x 40 g/mol) NaOH in 100 g of polymer. This corresponds to a weight percent of NaOH reactant incorporated of 2.64%.
2.7 Chemicals that are comparable to listed chemicals
If you’re introducing any of the chemicals in column B of the table below, your introduction could be categorised as exempted.
If your chemical is in the comparable chemicals table
If your chemical is in column B of the table, it means that it is comparable to a chemical that is already listed on the Inventory. Go to column C of the same row to find the chemical it is comparable to. Next, search for this chemical on the Inventory using the CAS number in column C to check whether there are any regulatory requirements or obligations for the listed chemical.
If your search results show:
- there are no regulatory requirements for the chemical, your introduction is categorised as exempted
- there are regulatory requirements for the chemical and you can meet these requirements, your introduction is categorised as exempted
- there are regulatory requirements for the chemical, but you cannot meet these requirements and none of the other introductions described on this page apply to you, move onto step 3: Introductions that are categorised as reported.
If your chemical is not in the comparable chemicals table
If your chemical is not in column B of the table below and none of the other introductions described on this page apply to you, move on to step 3: Introductions that are categorised as reported.
| A. Item | B. Comparable chemical (Industrial chemical to be introduced) | C. Industrial chemical already listed on the Inventory |
|---|---|---|
| 1 | Aloe barbadensis, extract CAS number: 94349-62-9 | Aloe vera, extract CAS number: 85507-69-3 |
| 2 | Brassica oleracea botrytis, extract CAS number: 223749-36-8 | Cabbage, extract CAS number: 89958-13-4 |
| 3 | Brassica oleracea, extract CAS number: 91771-39-0 | Cabbage, extract CAS number: 89958-13-4 |
| 4 | Brassica oleracea gemmifera, extract CAS number: 1174275-27-4 | Cabbage, extract CAS number: 89958-13-4 |
| 5 | Fatty acids, palm-oil, sodium salts CAS number: 61790-79-2 | Fatty acids, C14-18 and C16-18-unsaturated, sodium salts CAS number: 67701-11-5 |
| 6 | Jojoba, extract CAS number: 90045-98-0 | Jojoba oil CAS number: 61789-91-1 |
| 7 | 3,6,9,12,15,18,21,21,24,27-Nonaoxanonatriacontan-1-ol CAS number: 3055-99-0 | Poly(oxy 1,2-ethanediyl), α-dodecyl-ω-hydroxy CAS number: 9002-92-0 |
| 8 | Matricaria recutita, extract CAS number: 84082-60-0 | Oils, Chamomile, German CAS number: 8002-66-2 |
| 9 | Orange, extract CAS number: 84012-28-2 | Orange, sweet, extract CAS number: 8028-48-6 |
| 10 | Pelargonium roseum, extract CAS number: 90082-55-6 | Pelargonium graveolens, extract CAS number: 90082-51-2 |
| 11 | Soya lecithins CAS number: 8030-76-0 | Lecithins CAS number: 8002-43-5 |
| 12 | Soya phospholipids CAS number: 308069-41-2 | Phospholipids CAS number: 123465-35-0 |
| 13 | Spiro[isobenzofuran- 1(3H),9’[9H]xanthen]-3-one, 2’,4’,5’,7’-tetrabromo -4,5,6,7-tetrachloro-3’,6’-dihydroxy-, aluminum salt (3:2) CAS number: 15876-58-1 | Spiro[isobenzofuran-1(3H),9’-[9H]xanthen]-3-one, 2’,4’,5’,7’-tetrabromo-4,5,6,7-tetrachloro-3’,6’-dihydroxy-, aluminum salt (3:1) CAS number: 27532-17-8 |
| 14 | Tridymite CAS number: 15468-32-3 | Silica CAS number: 7631-86-9 |
| 15 | Wheat germ oil CAS number: 313258-61-6 | Oils, wheat CAS number: 68917-73-7 |
Examples
Your proposed introduction:
You plan to introduce ‘fatty acids, palm-oil, sodium salts’ (CAS No. 61790‑79‑2), which is not on the Inventory, but is in column B of the comparable chemicals table. In column C of the same row you find ‘fatty acids, C14‑18 and C16‑18‑unsaturated, sodium salts’ (CAS No. 67701‑11‑5), which means this chemical is comparable to your chemical and listed on the Inventory. You search this chemical (CAS No. 67701‑11‑5) on the Inventory and find there are no regulatory requirements associated with the introduction of this chemical. This means you can introduce your chemical (CAS No. 61790‑79‑2) as an exempted introduction if you're registered with us.
Your proposed introduction:
You plan to introduce 'soya phospholipids' (CAS No.308069‑41‑2) in end use products at a concentration level of 30%. Soya phospholipids is in column B of the comparable chemicals table. In column C of the same row, you find 'phospholipids' (CAS No. 123465‑35‑0) which means this chemical is comparable to your chemical and listed on the Inventory. You search this chemical (CAS No. 123465‑35‑0) on the Inventory and find there are regulatory conditions under the term ‘defined scope of assessment'. It says 'This chemical has been assessed as a component of dermal cosmetic products at concentrations no more than 20%. This chemical is not to be used in topical products intended for the eye'.
You don't meet this condition because you plan to use your chemical – soya phospholipids – at a concentration of 30% in end use products. This means your introduction is not categorised as exempted. If none of the other introductions described on this page apply to you, go to step 3: Introductions that are categorised as reported.
2.8 Chemicals resulting from non-functionalised surface treatment of listed chemicals
Your chemical introduction is categorised as exempted if the chemical is a non-functionalised surface-treated chemical resulting from a reaction of chemicals that are all listed on the Inventory. To be an exempted introduction, your chemical must meet all of the following criteria:
- it is the result of a reaction between 2 or more chemicals, all of which are listed on the Inventory
- the reaction to produce the chemical occurs at the surface of one of the chemicals (the substrate chemical) and the substrate chemical is listed on the Inventory
- it does not have any reactive functional groups that were not already on the substrate chemical before the reaction occurred
- it is not introduced as a solid or in a dispersion that consist of solid particles, in an unbound state or as an aggregate or agglomerate, where at least 50% (by number size distribution) of the particles have at least one external dimension in the particle size range of 1 to 100 nm.
Step 2 outcome
My introduction meets the criteria on this page.
This means that your introduction is in the 'exempted' category.
Skip to Your obligations after categorisation to learn about your reporting and record-keeping obligations.
My introduction does not meet criteria on this page
If your introduction is not covered on this page, go to step 3.
Next – Step 3: Introductions that are categorised as reported
Step 3: Introductions that are categorised as reported
You can also use our Step 3 decision tool to help you complete this step.
3.1: Introductions of 10 kg or less in an AICIS registration year (1 September to 31 August)
Q1. Is the total introduction volume of the chemical 10 kg or less per year?
This is the combined volume of the chemical that you will introduce in an AICIS registration year in all your products that contain the chemical.
If yes, go to Q2. If no, go to 3.2: low-risk flavour or fragrance blend introductions, or skip to Step 4.
Q2. Is your chemical in this table below?
| Chemical name | CAS number |
| Benzene, 1,1'-(1,2-ethanediyl)bis[2,3,4,5,6-pentabromo- (also known as decabromodiphenylethane or DBDPE) | 84852-53-9 |
| Benzene, 1,1'-oxybis-, pentabromo derivative (also known as pentabromodiphenyl ether) | 32534-81-9 |
| Benzene, 1,2,3,4,5-pentachloro- | 608-93-5 |
| Benzene, hexachloro- | 118-74-1 |
If no, go to Q3.
If yes, the chemical is not eligible for the reported category - introductions of 10 kg or less. The reason is because the chemical was either removed from the AICIS Inventory, or its certificate was cancelled because risks to human health or the environment could not be managed. Go to 3.2: low-risk flavour or fragrance blend introductions or skip to Step 4.
Q3. Will your chemical have an end use in cosmetics?
Note: if your chemical will be an ingredient in cosmetic products, then answer yes.
If yes, go to Q4. If no, skip to Q5.
Q4. Is your chemical prohibited or restricted in the EU or USA for use as a cosmetic or in a cosmetic?
Chemicals that are prohibited or restricted in the European Union (EU) or the United States of America (USA):
- Annex II of Regulation 1223/2009/EC on Cosmetic Products (prohibited substances)
- Annex III of Regulation 1223/2009/EC on Cosmetic Products (restricted substances)
- US Federal Food, Drug & Cosmetic Act (prohibited and restricted ingredients in cosmetics)
If no, go to Q5. If yes, go to 3.2: low-risk flavour or fragrance blend introductions or skip to Step 4.
Q5. As far as you know, is the chemical classified according to the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) as having:
- carcinogenicity?
- germ cell mutagenicity?
- reproductive toxicity?
Note: You could check the SDS, product information sheets, or find out from your supplier.
If no to all, go to Q6. If yes to one or more, go to 3.2: low-risk flavour or fragrance blend introductions or skip to Step 4.
Q6. As far as you know, will the chemical be introduced as a solid or in a dispersion?
Note: You could find out about the appearance from the SDS, technical data sheet or your supplier. For example, if the SDS indicates that the chemical being introduced is a liquid, answer 'no'.
If yes or unsure, go to Q7. If no, skip to Q8.
Q7. As far as you know, does the chemical consist of solid particles, in an unbound state or as an aggregate or agglomerate, any of which have at least one external dimension in the nanoscale (1-100 nm)?
Note: You could check if there are any claims related to the presence of solid particles at the nanoscale in technical data sheets and commercial labels for the product containing the chemical.
If no, go to Q8. If yes, go to 3.2: low-risk flavour or fragrance blend introductions or skip to Step 4.
Q8. Does statement A) or B) apply about your chemical?
- As far as you know, the chemical does not contain fluorine. You could check the chemical name or INCI name to see if it indicates that the chemical contains fluorine (F), or you could check with your supplier.
- The chemical is an inorganic salt.
If A) or B) or both apply, go to Q9. If neither apply, go to 3.2: low-risk flavour or fragrance blend introductions or skip to Step 4.
Q9. As far as you know, is your chemical persistent, bioaccumulative and toxic (PBT) under the Australian environmental criteria for PBT?
Tip: You could check the chemical’s SDS or find out from your supplier. Further information on the meaning of PBT is in the ‘Australian Environmental Criteria for Persistent, Bioaccumulative and/or Toxic Chemicals’ on the Department of Climate Change, Energy, the Environment and Water’s website.
If yes, go to 3.2: low-risk flavour or fragrance blend introductions or skip to Step 4.
Optional: you can continue through steps 4, 5 and 6 to see if your introduction can be in the exempted category.
3.2 Low-risk flavour or fragrance blend introductions
Flavour blends are mixtures of chemicals that are formulated to impart a taste. Fragrance blends are mixtures of chemicals that are formulated to impart a scent or cover a malodour.
Your introduction is categorised as reported if it meets requirements of either Scenario 1 or Scenario 2.
Scenario 1
Do all 6 points apply to your chemical?
- Your chemical is part of a flavour or a fragrance blend and the blend is introduced either on its own, or with other chemicals.
- The concentration of your chemical when it is introduced is 1% or less.
- The concentration of your chemical in end-use products is 1% or less.
- Your chemical does not have any of these hazard characteristics in the highest human health hazard band (hazard band C):
- is an inorganic arsenic compound
- contains beryllium, cadmium, chromium (VI), lead or nickel
- carcinogenicity
- reproductive toxicity
- developmental toxicity
- adverse effects mediated by an endocrine mode of action
- genetic toxicity
- Your chemical does not have any of these hazard characteristics in the highest environment hazard band (hazard band D):
- contains arsenic, cadmium, lead or mercury
- ozone depleting chemical
- synthetic greenhouse gas
- adverse effects mediated by an endocrine mode of action
- persistent, bioaccumulative, toxic
- Your chemical must either be on the IFRA Transparency List at the time that your pre-introduction report is submitted, or certain information about its introduction must be given to us before you introduce the chemical, including:
- the CAS number (if assigned)
- CAS name, the IUPAC name, or an eligible INCI plant extract name
- information on known hazard characteristics
- the maximum concentration of the chemical in the blend at introduction and end use
- the name you use to refer to the blend.
If you answered no to any of the points in Scenario 1, check Scenario 2.
If you answered yes to all points in Scenario 1, then your introduction is categorised as reported (low risk flavour or fragrance blend introductions). Skip to Your obligations after categorisation.
Optional: you can continue through steps 4, 5 and 6 to see if your introduction can be in the exempted category.
Scenario 2
Do all of the following points apply to your chemical introduction?
- Your chemical is part of a flavour or fragrance blend and the blend will be introduced either on its own, or with other chemicals.
- Either A) or B) applies:
- The total volume of the chemical that you will import or manufacture in an AICIS registration year will be 1,000 kg or less, plus both:
- concentration of the chemical at introduction is 1 % or less
- concentration of the chemical in end-use products is 1 % or less.
- The total volume of chemical that you will import or manufacture in an AICIS registration year will be 10 kg or less.
- The total volume of the chemical that you will import or manufacture in an AICIS registration year will be 1,000 kg or less, plus both:
- As far as you (the introducer) know, your chemical is not one of these GHS hazard classes:
- germ cell mutagenicity
- carcinogenicity
- reproductive toxicity.
- As far as you (the introducer) know, your chemical is not persistent, bioaccumulative and toxic (PBT) under the Australian environmental criteria for PBT. To answer this, check if your chemical meets the ‘Australian Environmental Criteria for Persistent, Bioaccumulative and/or Toxic Chemicals’ on the Department of Climate Change, Energy, the Environment and Water’s website.
- As far as you (the introducer) know, your chemical does not cause adverse effects mediated by an endocrine mode of action.
Further information on the meaning of adverse effects mediated by an endocrine mode of action is in the Industrial Chemicals Categorisation Guidelines and also in this guide – see human health hazard band C and environment hazard band D. - Your chemical is either A) or B)
- Your chemical is on the IFRA Transparency List at the time that you submit your pre-introduction report for introduction of the chemical.
- You, or someone else, will provide the following information before you introduce the chemical:
- CAS number (if assigned).
- CAS name, the IUPAC name, or an eligible INCI plant extract name
- Any hazard characteristics of the chemical that you, or the person providing the information knows about.
- The maximum concentration of the chemical in the blend at both introduction and end use.
- Name of the blend.
- You will use the chemical in accordance with IFRA Standards (which can include limits or set criteria for certain chemicals).
If you answered no to any of the points in Scenario 2, then go to 3.3 Chemicals that are used only for research and development, or skip to Step 4.
If you answered yes to all points in Scenario 2, then your introduction is categorised as reported (low risk flavour or fragrance blend introductions). Skip to Your obligations after categorisation.
Optional: you can continue through steps 4, 5 and 6 to see if your introduction can be in the exempted category.
3.3 Chemicals that are used only for research and development
Your introduction is categorised as reported if all of the following apply:
- you only use your chemical for research and development, or make it available to another person who only uses it in research and development
- you don’t make your chemical available to the public in any form (whether on its own, in combination with other industrial chemicals or as part of an article)
- you use control measures to eliminate or minimise any risks to the environment and any risks to the people involved in using the chemical for research and development
and point 1 or 2 or 3 applies:
- you will introduce more than 250 kg of your chemical in a registration year, use of your chemical will be subject to your (the introducer’s) control and you can demonstrate that all quantities of your chemical are not introduced as a solid or in a dispersion. To prove that your chemical is not introduced as a solid or in a dispersion, you might have an SDS or product information sheet that indicates the appearance (for example, in liquid form).
- you will introduce more than 250 kg of your chemical in a registration year, use of your chemical will be subject to your (the introducer’s) control and you can demonstrate that all quantities of your chemical do not consist of solid particles in an unbound state or as an aggregate or agglomerate, where at least 50% (by number size distribution) of the particles have at least one external dimension in the particle size range of 1 to 100 nm. To prove that your chemical does not consist of solid particles in an unbound state or as an aggregate or agglomerate, where at least 50% (by number size distribution) of the particles have at least one external dimension in the particle size range of 1 to 100 nm, you might have a study report about the particle size distribution of your chemical.
- you will introduce more than 10 kg, but not more than 100 kg of your chemical in a registration year.
Read our extra guidance on categorisation of chemicals introduced for research and development
If your introduction does not meet the criteria, then go to Step 4.
Optional: you can continue through steps 4, 5 and 6 to see if your introduction can be in the exempted category.
Step 3 outcome
My introduction meets the criteria on this page
This means that your introduction is in the 'reported' category. Skip to 'Your obligations after categorisation' to learn about your reporting and record-keeping obligations.
Optional: You can continue through steps 4, 5 and 6 to see if your introduction can be in the exempted category. If you would like your chemical to be added to the Inventory, you can apply for an assessment certificate (fee applies).
My introduction does not meet the criteria on this page
Continue with this guide to work out if your introduction is in the exempted, reported or assessed category. Go to Step 4: Work out your introduction's risk to human health.
Step 4: Work out your introduction's risk to human health
In Step 4, you need to work out the human health risk of your introduction - is it medium to high, low or very low? To work this out start at 4.1 and continue as far as you need to through each step.
Once you have your answer for human health, move to step 5 to work out the risk to the environment of your introduction.
At Step 6, you'll combine the human health risk and environment risk for the final category of your introduction.
To be able to finish your categorisation you need to work out the risks of your introduction to human health and the environment.
Step 4.1 Introductions that are always medium to high risk for human health
Instructions
Go through A, B and C to work out if you are, or are not, introducing any of these types of chemicals. You must keep records of study reports and other information that you used to answer each question.
B. Is your chemical a certain polyhalogenated organic chemical?.
C. Is your chemical a certain chemical at the nanoscale?.
_______________________________________________________________________________________________________________________________
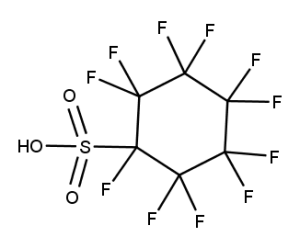
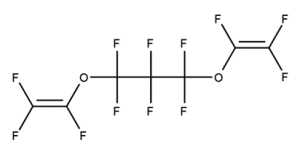
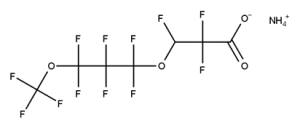
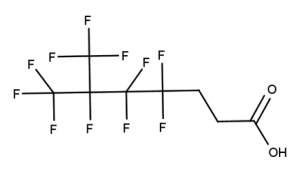
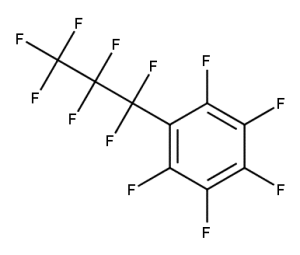
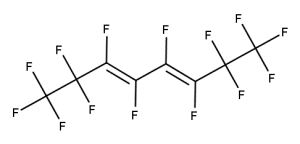
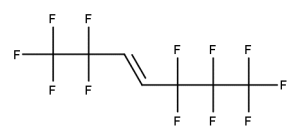
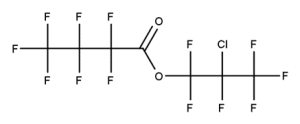
A. Is your chemical a designated fluorinated chemical (including per- and polyfluoroalkyl substances, known as PFAS)?
Fluorinated chemicals contain fluorine atoms and include per- and polyfluoroalkyl substances (PFAS). These are commonly used in products to add resistance to heat, other chemicals, and abrasion. They also act as dispersion, wetting or surface treatment agents. We have an increased level of concern for introductions of designated fluorinated chemicals (including PFAS), because these chemicals, or their degradation products, may be persistent in the environment, bioaccumulate and be highly toxic.
A designated fluorinated chemical is an industrial chemical that contains a sequence of atoms (whether linear, branched or cyclic) to which all of the following applies:
- subject to paragraph (b), the sequence consists only of at least 4, but no more than 20, fluorinated carbon atoms, none of which are fluorinated carbon atoms that are part of conjugated double bonds;
- if the sequence is broken in any place, the break consists only of a single atom or single substituted atom;
- the sequence includes at least one perfluorinated methyl group (CF3) or perfluorinated methylene group (CF2).
Fluorinated carbon atom means a carbon atom attached to at least one fluorine atom.
For a chemical to meet the definition of a ‘designated fluorinated chemical’:
• the sequence of carbon atoms can be linear, branched, or cyclic.
• the sequence must consist only of at least 4, but maximum 20 fluorinated carbon atoms.
• the sequence can include multiple breaks by a single atom (such as O or S) or substituted atom (such as C=O)
• the chemical can be polyfluorinated, provided the sequence contains at least one perfluorinated methyl group (CF3) or perfluorinated methylene group (CF2)
• fluorinated carbon atoms that are part of conjugated double bonds are not counted as part of the sequence.
Conjugated double bonds could include aromatic carbons.
We have extra guidance on categorisation of fluorinated chemicals
Some introductions are always medium to high risk to human health. This means they will be in the assessed introduction category and you need to apply for an assessment certificate.
Example 1
CAS number: 13252-13-6
Notes about this chemical:
- Sequence of 5 fluorinated carbon atoms.
- Ether linkage is a single atom break in the sequence.
Example 2
CAS number: 2106-55-0
Notes about this chemical:
- Sequence of 6 fluorinated carbon atoms.
- Sequence can be cyclic.
Example 3
CAS number: 13846-22-5
Notes about this chemical:
- Sequence of 7 fluorinated carbon atoms.
- Fluorinated carbon atoms on double bond considered part of sequence as double bond is not conjugated.
- Ether linkages considered a single atom break in the sequence.
- Multiple single atom or single substituted atom breaks allowed in the sequence.
Example 4
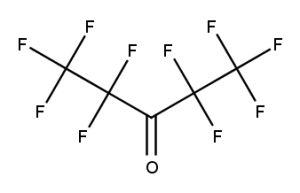
CAS number: 684-32-2
Notes about this chemical:
- Sequence of 4 fluorinated carbon atoms.
- Carbon atom of carbonyl group considered a single substituted atom break in the sequence.
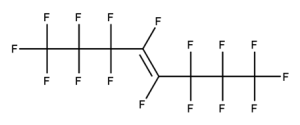
Example 5
CAS number: 115264-42-1
Notes about this chemical:
- Sequence of 8 fluorinated carbon atoms.
- Fluorinated carbon atoms on double bond considered part of sequence as the double bond is not conjugated.
Example 6
CAS number: 958445-44-8
Notes about this chemical:
- Sequence of 6 fluorinated carbon atoms.
- “CFH” carbon atom included in sequence.
- Ether linkages considered a single atom break in the sequence.
- Multiple single atom or single substituted atom breaks allowed in the sequence.
Example 7
CAS number: 207987-90-4
Notes about this chemical:
- Sequence of 5 fluorinated carbon atoms.
- Sequence can be branched.
Example 1
CAS number: 54326-26-0
Notes about this chemical:
- Sequence of 3 fluorinated carbon atoms
- Fluorinated carbon atoms on aromatic ring excluded from sequence
Example 2
CAS number: 105311-63-5
Notes about this chemical:
- Sequence of 2 fluorinated carbon atoms
- Sequence is broken by fluorinated carbon atoms that are part of conjugated double bonds
Example 3
CAS number: 2511100-75-5
Notes about this chemical:
- Sequence of 3 fluorinated carbon atoms
- Sequence is broken by more than a single atom or single substituted atom
Example 4
CAS number: 67135-90-4
Notes about this chemical:
- Sequence of 3 fluorinated carbon atoms
- Sequence broken by more than a single atom or single substituted atom
No I am not introducing a designated fluorinated chemical
You must have information about your chemical's identity as proof that you're not introducing this type of chemical. You (or the chemical identity holder) need to provide the information if we ask for it.
Next step: Go to 'B. Is your chemical a certain polyhalogenated organic chemical?'.
Yes I am introducing a designated fluorinated chemical
Outcome: Your introduction is medium to high indicative risk to both human health and the environment. This means your introduction is in the assessed category and called an 'assessed introduction'.
- Before you can introduce the chemical, you must apply for an assessment certificate and select 'Health and environment focus' as the application type or apply for a commercial evaluation authorisation (if you meet the strict criteria).
- When you apply for an assessment certificate, you need to answer ‘yes’ when we ask if your introduction is a specified class of introduction. When we receive your application, we’ll contact you to ask for extra information that we need to assess the risks of your introduction.
__________________________________________________________________________________________________________________________________
B. Is your chemical a certain polyhalogenated organic chemical?
Polyhalogenated organic chemicals are carbon-based chemicals that contain more than one covalently bonded halogen atom, such as bromine, chlorine, fluorine, or iodine. Polyhalogenated organic chemicals are commonly used as flame retardants in plastics, textiles, and electronic circuitry. They may have long-term effects on human health and the environment. We have an increased level of concern for introductions of chemicals that are polyhalogenated organic chemicals because these chemicals, or their degradation products, may be persistent in the environment, bioaccumulate and be highly toxic.
No I am not introducing this type of chemical
You must have information about your chemical's identity as proof that you're not introducing this type of chemical. You (or the chemical identity holder) need to provide the information if we ask for it.
Next step: Go to 'C. Is your chemical a certain chemical at the nanoscale?' below.
Yes I am introducing this type of chemical
All introductions of polyhalogenated chemicals are specified class of introduction.
If the chemical identity information that you (or the chemical identity holder) have confirms you are introducing this type of chemical, you must consider which of the following circumstances apply to your introduction.
1. Polyhalogenated organic chemicals introduced at volumes less than or equal to 100 kg each year
Next step: Go to 'C. Is your chemical a certain chemical at the nanoscale?' below.
2. Polyhalogenated organic chemicals introduced at volumes higher than 100 kg each year
You need to have test results about the persistence of your chemical and any of its known environmental degradation products.
- Known environmental degradation products refer to the expected breakdown products of the chemical under environmentally relevant conditions. These breakdown products are ones that have been found in studies or reported in scientific literature.
- A persistent chemical remains intact in the environment for long periods of time. A chemical is persistent if its degradation half-life (T1/2) is greater than or equal to:
- 2 days in air or
- 2 months in water or
- 6 months in soil or
- 6 months in sediment.
To prove that your chemical and any of its known environmental degradation products are not persistent, we accept study results in option 1 or 2.
Option 1
A study conducted following an acceptable test guideline for ready biodegradability that results in the pass levels being reached within one of the following time periods:
- specified time period – such that the chemical is considered to be readily biodegradable or
- duration of the test – but not within the specified time period for the chemical to be considered readily biodegradable, provided biodegradation has started within the specified time period
If you have this study showing these results, then move on to 'C. Is your chemical a certain chemical at the nanoscale?' below.
Option 2
A study conducted following an acceptable test guideline for Transformation in Aquatic Sediment Systems that results in both a degradation half-life of less than 2 months in water and 6 months in sediment.
If you have this study showing these results, then move on to 'C. Is your chemical a certain chemical at the nanoscale?' below.
If you do not have either of the study results described in option 1 or 2
Outcome: Your introduction is medium to high indicative risk to human health and the environment because you cannot prove that your chemical (and any of its known environmental degradation products) are not persistent. This means your introduction is in the assessed category and called an 'assessed introduction'.
- Before you can introduce the chemical, you must apply for an assessment certificate and select 'Health and environment focus' as the application type, or apply for a commercial evaluation authorisation (if you meet the strict criteria).
- When you apply for an assessment certificate, you need to answer ‘yes’ when we ask if your introduction is a specified class of introduction. When we receive your application, we’ll contact you to ask for extra information that we need to assess the risks of your introduction.
______________________________________________________________________________________________________________________________
C. Is your chemical a certain chemical at the nanoscale?
Introductions of chemicals that meet all 4 criteria below are medium to high indicative risk to both human health and the environment. We refer to these introductions as 'certain chemicals at the nanoscale'. We have an increased level of concern for chemicals at the nanoscale, because of uncertainty about the risks of some of these chemicals due to their potentially different properties, such as chemical reactivity, relative to the non-nanoscale forms of the chemicals.
- It is introduced as a solid or is in a dispersion.
- It consists of solid particles in an unbound state or as an aggregate or agglomerate. At least 50% (by number size distribution) of the particles have at least 1 external dimension in the particle size range of 1nm to 100nm (ie. the nanoscale). Note that if you meet criteria 1 and 2, and regardless of whether you meet criteria 3 and 4, your introduction is a specified class of introduction.
- It is not soluble. This means the solubility of the chemical in water is less than 33.3 g/L measured following OECD test guideline 105 or 120 for water solubility; or the dissolution rate of the chemical is not more than 70%.
- The introduction of the nanoscale portion of the chemical (the part that has a particle size range of 1nm to 100nm) is not incidental to the introduction of the non-nanoscale portion. This is the case if any of the following apply:
- the manufacture of the chemical (in Australia or overseas) at the nanoscale is the result of a deliberate manufacturing decision
- the manufacture of the chemical (in Australia or overseas) at the nanoscale is necessary for the manufacture of the non-nanoscale portion of the chemical. This means that to make the non-nanoscale chemical, part of the chemical has to be at the nanoscale
- the chemical at the nanoscale has specific technical characteristics that are the intended result of changes in the manufacturing process. For example, if the process of manufacturing the chemical changes in order to change the particle size of the chemical, or its properties at the nanoscale. This could happen by:
- mechanical actions like milling, grinding, shearing, sieving or sonication
- chemicals reactions like electrochemical exfoliation, or catalysts
- other changes such as changes to pressure or temperature or pH or solvent
Yes I am introducing this type of chemical
This means that your introduction meets all 4 criteria above and is a 'certain chemical at the nanoscale'.
Outcome: Your introduction has a medium to high indicative risk to both human health and the environment. This means your introduction is in the assessed category and called an ‘assessed introduction’.
- Before you can introduce the chemical, you must apply for an assessment certificate and select 'Health and environment focus' as the application type or apply for a commercial evaluation authorisation (if you meet the strict criteria).
- When you apply for an assessment certificate, you need to answer ‘yes’ when we ask if your introduction is a specified class of introduction. When we receive your application, we’ll contact you to ask for extra information that we need to assess the risks of your introduction.
No I am not introducing this type of chemical
This means that you have information or studies to prove that your chemical does not meet any of the 4 criteria, or it only meets some of the 4 criteria. Answering the questions below will help you prove this. As you go through the questions, we'll tell you the next steps you should take.
Definition - specified class of introduction
A ‘specified class of introduction’ are introductions that have an increased level of concern to human health or the environment. The reason is due to greater potential for certain hazards or high level of human or environmental exposure. Additional, or different, requirements relating to hazard information, reporting or record keeping apply to specified classes of introductions. These vary depending on whether you have categorised your introduction as exempted, reported or assessed.
Next – Step 4.2 Introductions that can be low risk for human health
Step 4.2 Introductions that can be low risk for human health
This step relates to introductions that are internationally assessed for human health. These must meet all of the following criteria to be considered ‘low indicative risk’ for human health.
Skip this step if you are not using an internationally assessed chemical.
Note: Your introduction might still be low indicative risk for human health but you will need to complete steps 4.3, 4.4 and 4.5 to work this out.
Step 4.2.1
Refer to our Guide to categorising internationally assessed introductions. It has extra information for introducers using international assessments and covers scenarios and outcomes for chemicals that are internationally assessed for:
- human health only
- the environment only
- both human health and the environment
It also lists the trusted overseas bodies we accept assessments from.
The relevant section to refer to in the guide to help you complete step 4.2 is Internationally assessed for human health only.
Step 4.2.2
Once you’ve read the guide to categorising internationally assessed introductions, you'll be able to work out whether your introduction either:
- meets our criteria for internationally assessed for human health
- does not meet our criteria for internationally assessed for human health
Your introduction meets our criteria for internationally assessed for human health
Option 1
- Keep the outcome you already have — your introduction is low risk for human health; and
- Go to step 5 to start categorising your introduction's indicative risk for the environment.
Option 2
Check to see if your introduction can be very low risk for human health by completing the rest of step 4:
- Complete Step 4.3 — Work out your introduction's human health exposure band; then
- Complete Step 4.4 — Work out your introduction's human health hazard characteristics
Once you’ve done this, go to step 4.5 for your final answer.
Your introduction does not meet our criteria for internationally assessed for human health
Continue with step 4 to work out your introduction's risk for human health.
Next:
- Complete Step 4.3 — Work out your introduction's human health exposure band; then
- Complete Step 4.4 — Work out your introduction's human health hazard characteristics
Once you’ve done this, go to step 4.5 for your final answer.
If you've established your introduction is NOT medium to high risk for human health (Step 4.1), now see if your introduction CAN be low risk for human health.
Step 4.3 Work out your human health exposure band
Why do you need to work out your introduction's exposure band?
It is part of the process to identify the indicative human health risk of your introduction. In step 5, you also have to work out your introduction's environment exposure band.
What does a human health exposure band identify about your introduction?
It identifies the likelihood and extent of human exposure to the chemical. This likelihood and extent of exposure increases with each band. Exposure band 4 is the highest exposure band. Introductions in human health exposure band 4 will have the highest level of human exposure.
Information that's used to assign a chemical to its correct exposure band
The information you need to be able to work out your exposure band can be different depending on the exposure band criteria you will be using. Some of the exposure band criteria mainly depend on human health categorisation volume, while others mainly depend on the concentration of your chemical when it's introduced into Australia and during its end use. This is a full list of the information you might need to be able to work out your human health exposure band:
- Human health categorisation volume (needed for scenario 1 of Exposure Band 2, scenario 1 of Exposure Band 3 and Exposure Band 4) - use this guidance to help you calculate human health categorisation volume
- Concentration of your chemical at introduction (needed for Exposure Band 1, scenario 2 of Exposure Band 2 and scenario 2 of Exposure Band 3).
- Concentration of your chemical across all end uses (needed for Exposure Band 1, scenario 2 of Exposure Band 2 and scenario 2 of Exposure Band 3).
- If it has an end use in tattoo inks (which is always in human health exposure band 4)
- If there are any consumer end uses for the chemical introduction (needed for Exposure Band 1 and scenario 2 of Exposure Band 2 and).
Are you introducing a chemical with an end use in tattoo inks?
If yes, your introduction is in human health exposure band 4 – skip to Step 4.4: Work out your human health hazard characteristics.
If no, continue below to work out which exposure band (1, 2, 3 or 4) applies to your introduction.
Work out your human health exposure band
Exposure band 1 criteria
Your introduction is in exposure band 1 if it is either Scenario 1 or Scenario 2.
Scenario 1 – not for consumer end use and less than 0.1% concentration
Your introduction must meet all criteria below:
- The concentration of your chemical at introduction is less than 0.1 %.
- The concentration of your chemical is less than 0.1 % across all your introduction’s end uses.
- Your introduction is not for any consumer end use.
If you meet all criteria for Scenario 1, your introduction is in exposure band 1 for human health. Next, choose between option 1 and option 2:
Option 1: The indicative risk of your introduction to human health is low risk. You can skip the remainder of step 4 and go to step 5 to work out your introduction’s risk to the environment. Once you have completed step 5, you will be able to work out your introduction category at step 6.
Option 2: You can choose to continue with the rest of step 4 to see if your introduction can be very low risk to human health. To do this, work through Step 4.4: Work out your human health hazard characteristics, followed by step 4.5. You must then complete step 5 to work out your introduction’s risk to the environment.
---------------------------------------------------
If your introduction is not scenario 1, go to scenario 2 or exposure band 2 criteria.
Scenario 2 – controlled introduction and use of a chemical
Your introduction must meet all criteria below:
- Your introduction of the industrial chemical is not of an industrial chemical that has an end use in tattoo inks.
- The human health categorisation volume of your chemical does not exceed 25 kg.
- Your introduction is not for any consumer end use.
- During introduction and use of your chemical, you will implement one or both control measures:
1. isolate the industrial chemical from any person who could be exposed to it
2. use engineering controls to eliminate or minimise exposure to people, including a mechanical device or process.- If people could still be exposed to the chemical even after implementing the control measures above, then you will implement other control measures to minimise potential exposure as far as reasonably practicable. This must include workers wearing suitable personal protective equipment that you provide.
- You (the introducer) have full control of the industrial chemical.
See example below of a controlled introduction and use of a chemical that meets criteria for human health exposure band 1.
If you meet criteria for Scenario 2, your introduction is in exposure band 1 for human health. Next, choose between option 1 and option 2:
Option 1: The indicative risk of your introduction to human health is low risk. You can skip the remainder of step 4 and go to step 5 to work out your introduction’s risk to the environment. Once you have completed step 5, you will be able to work out your introduction category at step 6.
Option 2: You can choose to continue with the rest of step 4 to see if your introduction can be very low risk to human health. To do this, work through Step 4.4: Work out your human health hazard characteristics, followed by step 4.5. You must then complete step 5 to work out your introduction’s risk to the environment.
---------------------------------------------------
If your introduction is not scenario 2, go to the exposure band 2 criteria.
Exposure Band 2 criteria
Your introduction is in exposure band 2 if it is either Scenario 1 or Scenario 2:
Scenario 1
The human health categorisation volume of your chemical does not exceed 25kg.
Scenario 2
Your introduction must meet all of the following criteria:
- The concentration of your chemical at introduction is less than 0.1 %.
- The concentration is less than 0.1 % across all your introduction’s end uses.
- The introduction has at least 1 consumer end use.
If you meet criteria for either Scenario 1 or 2, your introduction is in exposure band 2 for human health – skip to Step 4.4: Work out your human health hazard characteristics.
Otherwise, go to Exposure band 3 criteria.
Exposure band 3 criteria
Your introduction is in exposure band 3 if it is either Scenario 1 or Scenario 2:
Scenario 1
- The human health categorisation volume for your chemical does not exceed 100 kg - use this guidance to calculate.
Scenario 2
You must meet all following criteria:
- The concentration of your chemical at introduction is less than or equal to 1%.
- The concentration is less than or equal to 1% across all your introduction’s end uses .
If you meet criteria for Scenario 1 or 2, your introduction is in human health exposure band 3 – skip to Step 4.4: Work out your human health hazard characteristics.
Otherwise, go Exposure band 4 criteria.
Exposure band 4 criteria
Your introduction is in exposure band 4 if it is either Scenario 1 or Scenario 2:
Scenario 1
Your chemical will have an end use in tattoo inks.
Scenario 2
The human health categorisation volume for your introduction is greater than 100 kg.
If you meet criteria for Scenario 1 or 2, your introduction is in human health exposure band 4 – next, go to Step 4.4: Work out your human health hazard characteristics.
What is a 'consumer end use'?
Step 4.4 Work out your human health hazard characteristics
Important! Your starting point is always hazard band C (the highest hazard band)
Then work your way down the hazard bands (that is, C then B then A) to check if your chemical has any characteristics in these bands. After reading this page, go to human health hazard band C hazard characteristics.
You must have permission to use information that you relied on to demonstrate the absence of hazard characteristics. If we ask you for the information that you relied on to categorise your introduction, you need to provide us with the detailed information, including full study reports, of the kind we specify in this step to demonstrate the absence of the hazard characteristics.
Hazard characteristics in human health hazard bands
A chemical has a human health hazard characteristic if the chemical can cause damage, harm or adverse effects to humans. For example, a chemical that has the 'skin corrosion' hazard characteristic can cause irreversible damage to the skin of humans.
Human health hazard characteristics are split up into hazard bands. Hazard characteristics of most concern are in hazard band C, while those of lower concern are in hazard band A.
Our pages for human health hazard bands C, B and A describe hazard characteristics (eg carcinogenicity and so on) in each hazard band and the information you need to have to prove your chemical does not have a particular hazard characteristic.
Information you need and hazard characteristics you need to consider
This varies depending on your introduction’s human health exposure band.
If your introduction is in a lower exposure band
Generally, in the lower exposure bands, where the level of exposure to humans is relatively low, as a minimum you have to consider only a few hazard characteristics and you don’t need much information on them.
If your introduction is in a higher exposure band
In comparison, in higher exposure bands, where the level of exposure to humans is higher, generally you’ll need to consider more hazard characteristics and need more information on them.
Information you need for lower indicative risk
You will need more hazard information to be able to get to lower indicative risk outcomes. Generally, within any given human health exposure band you need:
- less hazard information to get to medium to high risk
- more hazard information to get to low risk
- the most hazard information to get to very low risk
See Step 4.5 for more information about indicative human health risk outcomes
Work out if your chemical has any hazard characteristics in human health hazard band A, B, C.
Where to start and when you can stop considering your chemical's hazard characteristics
You must consider each hazard characteristic in the hazard band you are in (unless there is a reason for you to stop sooner) — does your chemical have that hazard characteristic or not?
When you might not need to consider all of the hazard bands
- Because your introduction’s human health exposure band (which you worked out in step 4.3) doesn’t require it. For example, if your introduction’s human health exposure band is 1, you only need to consider the hazards in hazard band C to get to an indicative human health risk of either low or very low.
- Because the outcome for indicative human health risk that you are trying to get to doesn’t require it. For example, if your introduction’s human health exposure band is 3 and you want to get to an indicative human health risk of low, you only need to consider the hazard characteristics in human health hazard band C.
In many cases, you’ll only need to consider hazard band C. But in other cases you might need to consider C, B and A, because your introduction is in exposure band 3 or 4 and you are trying to get to very low indicative human health risk.
See step 4.5 for more about indicative human health risk outcomes
When you can stop working through your chemical’s human health hazard characteristics
Stop if you:
- determine that your chemical has a hazard characteristic in the hazard band (eg carcinogenicity — you are in hazard band C) or
- cannot demonstrate that your chemical does not have a certain hazard characteristic in that hazard band. This means that we consider your chemical to have this hazard characteristic or
- get to an indicative human health risk outcome and don’t want to go any further — see step 4.5 for more information about human health risk outcomes or
- have demonstrated that your chemical does not have any hazard characteristics in hazard bands C, B and A. This would only be needed for human health exposure bands 3 and 4. It means that the indicative human health risk of your introduction is very low.
After you stop, you don’t need to consider the remaining hazard characteristics in the hazard band where you stopped, or any of the hazard characteristics in lower hazard bands. Take note of where and why you stopped, then move to step 4.5.
Example: Anna's introduction is in human health exposure band 4. She considers all of the hazard characteristics in human health hazard band C and can demonstrate that her chemical does not have any of these hazards. Anna then moves on to hazard band B. She works through the hazard characteristics in this hazard band in the order that they are shown in the table. When Anna comes to eye damage, she finds that her chemical has the 'eye damage' hazard characteristic. This means Anna can stop there. The indicative human health risk of Anna's introduction is medium to high. She does not need to continue further to see if her chemical has any of the other hazard characteristics in hazard band B, like skin sensitisation, or specific target organ toxicity after repeated exposure. Also Anna doesn't need to consider if her chemical has any of the hazard characteristics in hazard band A, such as skin irritation.
How to consider each hazard characteristic
Look at whether your chemical meets the hazard characteristic definition based on the information that you have.
If it does meet the hazard characteristic definition, stop there and move to step 4.4.
If it does not meet the hazard characteristic definition, you’ll need to try and prove that your chemical does not have this hazard characteristic.
Our pages on hazard bands C, B and A describe hazard characteristics and the ways to prove that your chemical does not have a hazard characteristic.
How to prove that your chemical does not have a hazard characteristic
You can read about your options to prove that your chemical does not have a particular hazard characteristic on each human health hazard band page. These options include
- checking if your chemical is on the list of chemicals with high hazards for categorisation
- in silico predictions
- in vitro test results
- in vivo test results
- suitable read-across information in place of information on the chemical itself
- other information about your chemical that means that testing and in silico predictions are not necessary (that is, information waivers)
If you have access to existing information on the chemical or suitable read-across information, you should consider these first. If you need to generate new data to prove the absence of a hazard characteristic, you should choose non-animal test data when possible. You should only generate new animal test data as a last resort.
See our section on use of animal test data
If you can prove that your chemical does not have the hazard characteristic, move on to the next hazard characteristic in that hazard band, or from the next hazard band down.
If you cannot prove that your chemical does not have the hazard characteristic, stop there – your chemical is considered to have this hazard characteristic. Take note of the hazard band that this hazard characteristic is in and move on to step 4.5.
If your chemical is one of these:
- polyhalogenated organic chemical
- UV filter
- is introduced for an end use in a tattoo ink
- is introduced for an end use in an article that is a children’s toy or a children’s care product
- is introduced for an end use in an article with food contact
there may be different requirements for you to prove that your chemical does not have certain hazard characteristics.
In most cases we do not expect that you will have information about the high level hazard characteristics in human health hazard band C, such as carcinogenicity. Instead, to demonstrate that your chemical does not have these hazard characteristics you can search the list of chemicals with high hazards for categorisation. This is a list that we have compiled directly from trusted international sources, and provides you with a single place to search for your chemical to check if it might be known to have these hazards. Our hazard band pages tell you when you might need to search the high hazards list.
A list of resources to help you with this step
- List of chemicals with high hazards for categorisation
- In silico information- an overview of which human health and environment (step 5.4) characteristics have in silico options and which in silico models are appropriate.
- Acceptable test guidelines for each human health hazard characteristic listed in this step
- Suitable read across information
- Decision tools for step 4.4 (self-guided tools to help you categorise your introduction):
Human health hazard bands - what are the hazard characteristics in each hazard band?
The following pages describe the hazard characteristics in each hazard band and the information you need to have to prove your chemical does not have a particular hazard characteristic. Follow instructions on each of these pages. Always start with hazard band C.
Human health hazard band C hazard characteristics
Human health hazard characteristics are split into hazard bands. Hazard characteristics of most concern are in hazard band C, while those of lower concern are in hazard band A.
Getting started
Hazard band C has 7 hazard characteristics you need to consider:
- chemical is an inorganic arsenic compound
- chemical contains beryllium, cadmium, chromium (VI), lead, or nickel
- carcinogenicity
- reproductive toxicity
- developmental toxicity
- adverse effects mediated by an endocrine mode of action
- genetic toxicity
Work your way through each hazard characteristic on this page. Look at whether your chemical meets the hazard characteristic definition based on the information that you have.
If it does meet the hazard characteristic definition, stop there - your introduction's human health hazard band is C. Move on to the next step - step 4.5 Work out your human health risk for categorisation.
If it does not meet the hazard characteristic definition, you’ll need to prove that your chemical does not have this hazard characteristic. The information that you need to prove this for each hazard characteristic is shown below. If you do not have this information, stop there - your introduction’s human health hazard band is C. Move onto the next step – step 4.5 Work out your human health risk for categorisation.
If you do have this information (so you can prove that the chemical does not have the hazard characteristic), move onto the next hazard characteristic on this page.
After you have considered all the hazard characteristics on this page and have proven that the chemical does not have any of them, decide whether you can stop there or continue to human health hazard band B. This depends on the exposure band of your introduction.
If your introduction is in human health exposure band 1 or 2, stop here - you don’t need to consider any other hazard characteristics. Next go to step 4.5 to work out your human health risk for categorisation.
If your introduction is in human health exposure band 3 you can choose to stop (and go to step 4.5 to work out your human health risk for categorisation), or to continue to human health hazard band B and then A.
If your introduction is in human health exposure band 4, continue to human health hazard band B.
Detailed instructions about each human health hazard band C hazard characteristics you need to consider
Chemical is an inorganic arsenic compound
A chemical is an inorganic arsenic compound means both of these apply to the industrial chemical:
- contains arsenic and
- does not contain carbon.
There are no extra information requirements to prove that the chemical does not have this hazard characteristic.
Chemical contains beryllium, cadmium, chromium (VI), lead or nickel
Chemical contains beryllium, cadmium, chromium (VI), lead or nickel means that the industrial chemical contains one or more of the following chemical elements:
- beryllium
- cadmium
- chromium (VI)
- lead
- nickel
There are no extra information requirements to prove that the chemical does not have this hazard characteristic.
Carcinogenicity
Carcinogenicity means that any of the following apply to the industrial chemical:
- the chemical is a known, presumed or suspected human carcinogen, as described in chapter 3.6 of the GHS, with the chemical classified as carcinogenicity (category 1 or 2), or
- the chemical is on the list of chemicals with high hazards for categorisation based on its carcinogenicity, or
- the chemical is an ester or salt of specified chemicals in the table below, which are on the list of chemicals with high hazards for categorisation based on carcinogenicity, unless an exception as identified in the table below is met for that chemical, or
- an in vivo study on the chemical conducted following an acceptable test guideline for carcinogenicity, chronic toxicity, subchronic oral toxicity, subchronic dermal toxicity or subchronic inhalation toxicity results in the induction of cancer, or an increase in the incidence of cancer.
Information required to demonstrate the absence of the hazard characteristic, carcinogenicity
- Confirmation that the chemical is not on the list of chemicals with high hazards for categorisation, based on its carcinogenicity and
- Confirmation that the chemical is not an ester or salt of the specified chemicals in the table below, which are on the list of chemicals with high hazards for categorisation, based on carcinogenicity
Carcinogenicity - exception criteria
Check the following table – an ester or salt of the chemical has the carcinogenicity characteristic, unless one or more of the following exception criteria apply.
| CAS number | Chemical name | An ester or salt of the chemical has the carcinogenicity hazard characteristic, unless one or more of the below exception criteria apply |
|---|---|---|
| 108-78-1 | 1,3,5-Triazine-2,4,6-triamine (Melamine) |
|
| 139-13-9 | Glycine, N,N-bis(carboxymethyl)- |
|
| 90-43-7 | [1,1’-Biphenyl]-2-ol |
|
| 615-05-4 | 1,3-Benzenediamine, 4-methoxy- (diaminoanisole) |
|
Note – Low levels of low molecular weight species (in relation to a polymer) means less than 10% (by mass) of molecules with a molecular weight that is less than 500 g/mol and less than 25% (by mass) of molecules with a molecular weight that is less than 1,000 g/mol.
- In addition, if the human health exposure band for the introduction is 4 and the chemical is a UV filter, information is required to justify why the chemical would not cause carcinogenicity mediated by exposure to UV light. This may include one or more of the following:
- the chemical has a molar extinction/absorption coefficient of less than 1,000 Lmol- 1cm-1 at wavelengths between 290 and 700 nm (based on the results of a study following OECD test guideline 101), or
- results from in vitro phototoxicity studies, or
- results from in vivo carcinogenicity studies where the methods have been modified to include photoactivation.
More information: categorisation of UV filters
This page accompanies step 4.4 Work out your human health hazard characteristics.
Reproductive toxicity
Reproductive toxicity means that any of the following apply to the industrial chemical:
- the chemical is known, presumed or suspected to produce adverse effects on sexual function and fertility, as described in chapter 3.7 of the GHS, with the chemical classified as toxic to reproduction (category 1 or 2), or
- the chemical is on the list of chemicals with high hazards for categorisation based on its reproductive toxicity, or
- the chemical is an ester or salt of specified chemicals in the table below, which are on the list of chemicals with high hazards for categorisation based on reproductive toxicity, unless an exception as identified in the table below is met for that chemical, or
- an in vivo study on the chemical conducted following an acceptable test guideline for reproductive toxicity, carcinogenicity, chronic toxicity, subchronic oral toxicity, subchronic dermal toxicity or subchronic inhalation toxicity results in adverse effects on sexual function and fertility, as described in chapter 3.7 of the GHS.
Information required to demonstrate the absence of the hazard characteristic, reproductive toxicity
- If the chemical is a polyhalogenated organic chemical and the human health exposure band for the introduction is 4 -
- an in vivo test result on the chemical or suitable read across information conducted following an acceptable test guideline for reproductive toxicity, which results in none of the adverse effects on sexual function or fertility described in chapter 3.7 of the GHS;
- Otherwise –
- confirmation that the chemical is not on the list of chemicals with high hazards for categorisation, based on its reproductive toxicity
- confirmation that the chemical is not an ester or salt of the specified chemicals shown in the below table, which are on the list of chemicals with high hazards for categorisation based on reproductive toxicity.
Reproductive toxicity - exception criteria
Check the following table - an ester or salt of the chemical has the reproductive toxicity hazard characteristic, unless one or more of the following exception criteria apply.
| CAS name | Chemical name | An ester or salt of the chemical has the reproductive toxicity hazard characteristic, unless one or more of the below exception criteria apply |
|---|---|---|
| 110-80-5 | Ethanol, 2-ethoxy-, |
|
| 109-86-4 | Ethanol, 2-methoxy- |
|
| 98-73-7 | Benzoic acid, 4-(1,1-dimethylethyl)- |
|
| 97-99-4 | 2-Furanmethanol, tetrahydro- (tetrahydro-2-furylmethanol) |
|
| Various | Boric acid |
|
| 80-05-7 | Phenol, 4,4'-(1-methylethylidene)bis- (Bisphenol A) |
|
| 80-09-1 | Phenol, 4,4'-sulfonylbis- (Bisphenol S) |
|
| 98-54-4 | Phenol, 4-(1,1-dimethylethyl)- |
|
| Various | Nonylphenols on the list of chemicals with high hazards for categorisation – includes linear and branched isomers |
|
| Various | Dodecylphenols on the list of chemicals with high hazards for categorisation – includes linear and branched isomers |
|
Note – Low levels of low molecular weight species (in relation to a polymer) means less than 10% (by mass) of molecules with a molecular weight that is less than 500 g/mol and less than 25% (by mass) of molecules with a molecular weight that is less than 1,000 g/mol.
Developmental toxicity
Developmental toxicity means that any of the following apply to the industrial chemical:
- the chemical is known, presumed or suspected to produce adverse effects on the development of the offspring or effects on the offspring via lactation, as described in chapter 3.7 of the GHS, with the chemical classified as follows:
- toxic to reproduction (category 1 or 2), or
- effects on or via lactation, or
- the chemical is on the list of chemicals with high hazards for categorisation based on its developmental toxicity, or
- the chemical is an ester or salt of specified chemicals in the table below, which are on the list of chemicals with high hazards for categorisation based on developmental toxicity, unless an exception as identified in the table below is met for that chemical, or
- an in vivo study on the chemical conducted following an acceptable test guideline for developmental toxicity or reproductive toxicity results in adverse effects on the development of the offspring or effects on the offspring via lactation, as described in chapter 3.7 of the GHS.
Information required to demonstrate the absence of the hazard characteristic, developmental toxicity
- If the chemical is a polyhalogenated organic chemical and the human health exposure band for the introduction is 4 –
- an in vivo test result on the chemical or suitable read across information conducted following an acceptable test guideline for developmental toxicity or reproductive toxicity which results in none of the adverse effects on the development of the offspring or effects on the offspring via lactation, as described in chapter 3.7 of the GHS
- Otherwise –
- confirmation that the chemical is not on the list of chemicals with high hazards for categorisation, based on its developmental toxicity and
- confirmation that the chemical is not an ester or salt of the specified chemicals shown in the below table, which are on the list of chemicals with high hazards for categorisation, based on developmental toxicity.
Developmental toxicity - exception criteria
Check the following table - an ester or salt of the chemical has the development toxicity hazard characteristic, unless one or more of the following exception criteria apply.
| CAS number | Chemical name | An ester or salt of the chemical has the developmental toxicity hazard characteristic, unless one or more of the below exception criteria apply |
|---|---|---|
| 149-57-5 | Hexanoic acid, 2-ethyl (2-EHA) |
|
| 104-76-7 | 1-Hexanol, 2-ethyl- |
|
| 69-72-7 | Benzoic acid, 2-hydroxy- (salicylic acid) |
|
| 110-80-5 | Ethanol, 2-ethoxy-, |
|
| 109-86-4 | Ethanol, 2-methoxy- |
|
| 111-77-3 | Ethanol, 2-(2-methoxyethoxy)- |
|
| 97-99-4 | 2-Furanmethanol, tetrahydro- (tetrahydro-2-furylmethanol) |
|
| Various | Boric acid |
|
| 80-09-1 | Phenol, 4,4'-sulfonylbis- (Bisphenol S) |
|
| Various | Nonylphenols on the list of chemicals with high hazards for categorisation – includes linear and branched isomers |
|
Note – Low levels of low molecular weight species (in relation to a polymer) means less than 10% (by mass) of molecules with a molecular weight that is less than 500 g/mol and less than 25% (by mass) of molecules with a molecular weight that is less than 1,000 g/mol
Adverse effects mediated by an endocrine mode of action
Adverse effects of mediated by an endocrine mode of action means that any of the following apply to the industrial chemical:
- the chemical meets all of the following:
- it shows an adverse effect in an intact organism or its progeny, which is a change in the morphology, physiology, growth, development, reproduction or lifespan of an organism, system or (sub)population that results in an impairment of functional capacity, an impairment of the capacity to compensate for additional stress or an increase in the susceptibility to other influences, and
- it has an endocrine activity, which is the capacity to alter the function(s) of the endocrine system, and
- the adverse effect is a consequence of the endocrine activity
or
- the chemical is on the list of chemicals with high hazards for categorisation, based on its adverse effects mediated by an endocrine mode of action or
- the chemical is an ester or salt of specified chemicals in the table below, which are on the list of chemicals with high hazards for categorisation based on adverse effects mediated by an endocrine mode of action unless an exception, as identified in the table below, is met for that chemical, or
- the chemical meets all of the following:
- information is available that is relevant to determining whether the chemical has the hazard characteristic, adverse effects mediated by an endocrine mode of action, and
- the information has been considered in a weight of evidence analysis based on the following guidance documents:
- the EU guidance for identifying endocrine disruptors*, and
- the guidance provided in OECD GD 150**; and
- the weight of evidence analysis concludes that the chemical has the hazard characteristic, adverse effects mediated by an endocrine mode of action.
Information required to demonstrate the absence of the hazard characteristic, adverse effects mediated by an endocrine mode of action
- If the chemical has existing information relevant to determining whether it has the hazard characteristic, adverse effects mediated by an endocrine mode of action, information is required to demonstrate that the chemical does not have this hazard characteristic:
- this must involve a documented weight of evidence analysis based on the EU guidance for identifying endocrine disruptors* and the guidance in OECD GD 150**, and
- the analysis must conclude that the chemical does not have the hazard characteristic, adverse effects mediated by an endocrine mode of action.
- Otherwise–
- confirmation that the chemical is not on the list of chemicals with high hazards for categorisation, based on its adverse effects mediated by an endocrine mode of action and
- confirmation that the chemical is not an ester or salt of the specified chemicals shown in the below table, which are on the list of chemicals with high hazards for categorisation, based on adverse effects mediated by an endocrine mode of action.
Adverse effects mediated by an endocrine mode of action - exception criteria
Check the following table - an ester or salt of the chemical has the adverse effects mediated by an endocrine mode of action hazard characteristic, unless one or more of the following exception criteria apply.
| CAS number | Chemical name | An ester or salt of the chemical has the adverse effects mediated by an endocrine mode of action hazard characteristic, unless one or more of the below exception criteria apply |
|---|---|---|
| 80-05-7 | Phenol, 4,4'-(1-methylethylidene)bis- (Bisphenol A) |
|
| 80-09-1 | Phenol, 4,4'-sulfonylbis- (Bisphenol S) |
|
| Various | Dodecylphenols on the list of chemicals with high hazards for categorisation – includes linear and branched isomers |
|
Note – Low levels of low molecular weight species (in relation to a polymer) means less than 10% (by mass) of molecules with a molecular weight that is less than 500 g/mol and less than 25% (by mass) of molecules with a molecular weight that is less than 1,000 g/mol.
Genetic toxicity
Genetic toxicity means that any of the following apply to the industrial chemical:
- the chemical is known to induce or may induce mutations in the germ cells of humans, as described in chapter 3.5 of the GHS, with the chemical classified as germ cell mutagenicity (category 1 or 2), or
- the chemical is on the list of chemicals with high hazards for categorisation, based on its genetic toxicity, or
- the chemical is an ester or salt of specified chemicals in the table below, which are on the list of chemicals with high hazards for categorisation based on genetic toxicity unless an exception, as identified in the table below, is met for that chemical, or
- an in vitro study on the chemical:
- conducted following an acceptable test guideline for gene mutation or chromosomal abnormalities results in the prediction of mutagenic or genotoxic effects, as described in chapter 3.5 of the GHS, and
- the results of the study have not been negated by in vivo studies conducted on the chemical for gene mutation, chromosomal abnormalities or heritable germ cell mutagenicity, or
- an in vivo study on the chemical conducted following an acceptable test guideline for gene mutation, chromosomal abnormalities or heritable germ cell mutagenicity results in mutagenic or genotoxic effects, as described by chapter 3.5 of the GHS.
Information required to demonstrate the absence of the hazard characteristic, genetic toxicity
The information required to demonstrate that a chemical does not have the hazard characteristic, genetic toxicity, is:
- if the human health exposure band for the introduction is 4 - at least one of the following:
- information to demonstrate that the chemical is included on the Select Committee on GRAS Substances (SCOGS) Database as a Type 1 conclusion, and that the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- information to demonstrate that the chemical has been notified to the US FDA GRAS notification program and FDA had no questions about the notifier’s conclusion of GRAS status, and that the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- information that demonstrates that the chemical is a substance covered by Entry 9 of Annex V of the REACH Regulation, or
- information to demonstrate that the chemical is a high molecular weight polymer, and if you are seeking to demonstrate that the introduction meets the criteria for very low risk and is not one of the 'special cases' mentioned in step 4.5 - test results from an in vitro study on the polymer or from suitable read across information conducted following an acceptable test guideline for gene mutation, which demonstrates the absence of mutagenic effects, or
- test results that demonstrate the absence of mutagenic or genotoxic effects from both:
- study on the chemical or from suitable read across information conducted following an acceptable test guideline for gene mutation, and
- study on the chemical or from suitable read across information conducted following an acceptable test guideline for chromosomal abnormalities.
- if the human health exposure band for the introduction is 3, and you are seeking to demonstrate that the introduction meets the criteria for very low risk and is not one of the 'special cases' mentioned in step 4.5 - at least one of the following:
- inclusion of the chemical in the Select Committee on GRAS Substances (SCOGS) Database as a Type 1 conclusion, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- the chemical has been notified to the US FDA GRAS notification program and FDA had no questions about the notifier’s conclusion of GRAS status, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- information that demonstrates that the chemical is a substance covered by Entry 9 of Annex V of the REACH Regulation, or
- if the polymer is a high molecular weight polymer, test results from an in vitro study on the polymer or from suitable read across information conducted following an acceptable test guideline for gene mutations, which demonstrates the absence of mutagenic effects, or
- information that demonstrates the absence of mutagenic or genotoxic effects from both:
- information on the chemical or from suitable read across information that addresses gene mutations - this could be:
- a suitable in silico prediction, both with and without metabolic activation, or
- test results from a study conducted following an acceptable test guideline for gene mutations; and
- test results from a study on the chemical or from suitable read across information conducted following an acceptable test guideline for chromosomal abnormalities.
- information on the chemical or from suitable read across information that addresses gene mutations - this could be:
- Otherwise–
- confirmation that the chemical is not on the list of chemicals with high hazards for categorisation, based on its genetic toxicity and
- confirmation that the chemical is not an ester or salt of the specified chemicals shown in the below table, which are on the list of chemicals with high hazards for categorisation, based on genetic toxicity.
Note – Low levels of low molecular weight species (in relation to a polymer) means less than 10% (by mass) of molecules with a molecular weight that is less than 500 g/mol and less than 25% (by mass) of molecules with a molecular weight that is less than 1,000 g/mol.
Genetic toxicity - exception criteria
Check the following table - an ester or salt of the chemical has the genetic toxicity hazard characteristic, unless one or more of the following exception criteria apply.
| CAS No | Chemical name | An ester or salt of the chemical has the genetic toxicity hazard characteristic, unless one or more of the below exception criteria apply |
|---|---|---|
| 90-43-7 | [1,1’-Biphenyl]-2-ol |
|
| 615-05-4 | 1,3-Benzenediamine, 4-methoxy- (diaminoanisole) |
|
| 123-30-8 | Phenol, 4-amino- |
|
| 95-55-6 | Phenol, 2-amino- |
|
- in addition, if the human health exposure band for the introduction is 4 and the chemical is a UV filter, information is required to justify why the chemical would not cause genetic toxicity mediated by UV light. This may include one or more of the following:
- the chemical has a molar extinction coefficient/absorption coefficient of less than 1,000 Lmol-1cm-1 at wavelengths between 290 and 700 nm (based on the results of a study following OECD test guideline 101), or
- results from in vitro phototoxicity studies, or
- results from in vitro or in vivo genetic toxicity studies where the methods have been modified to include photoactivation.
More information: Categorisation of UV filters
Examples - checking exception criteria for esters or salts
Example 1 – chemical is a salt of a specified chemical which is on the list of chemicals with high hazards for categorisation, based on carcinogenicity
Peter wants to introduce the chemical glycine, N,N-bis[2-[bis(carboxymethyl)amino]ethyl]-, sodium salt (1:?) (CAS: 7578-43-0), which is a salt of Glycine, N,N-bis(carboxymethyl)- (CAS: 139-13-9) that is in the table of specified chemicals for carcinogenicity. The proposed introduction has a molecular weight of 415.33 g/mol and therefore does not meet the exception criterion “the salt/ester is a high molecular weight polymer, with low levels of low molecular weight species” nor the exception criterion “the molecular weight of the salt/ester is greater than or equal to 1,000 g/mol”. Thus, Glycine, N,N-bis(2-(bis(carboxymethyl)amino)ethyl)-, sodium salt (CAS: 7578-43-0) is considered to have the human health hazard characteristic carcinogenicity.
Peter’s introduction's human health hazard band is C so he should move on to the next step – step 4.5 Work out your human health risk for categorisation.
Example 2 – chemical is an ester of a specified chemical which is on the list of chemicals with high hazards for categorisation, based on carcinogenicity and genetic toxicityRose wants to introduce an ester of [1,1’-Biphenyl]-2-ol, which has a molecular weight greater than or equal to 1,000 g/mol. The ester itself is not on the List, but her chemical is an ester of [1,1’-Biphenyl]-2-ol, which is in the tables of specified chemicals for carcinogenicity and genetic toxicity. So she needs to consider whether an exception applies to this chemical. If an exception does not apply, the ester would be considered to have the same high hazard characteristics as [1,1’-Biphenyl]-2-ol (carcinogenicity and genetic toxicity). But because Rose’s chemical has a molecular weight greater than or equal to1,000 g/mol, it meets the exception criteria.
Rose’s ester is not considered to have the human health hazard characteristics of carcinogenicity and genetic toxicity.
Rose continues through step 4.4 of the categorisation process and next needs to follow our guidance on checking human health hazard band B hazard characteristics.
Example 3 – polymer is an ester of a specified chemical which is on the list of chemicals with high hazards for categorisation, based on genetic toxicityPaul wants to introduce a branched polymer containing phenol, 2-amino- bound to side chains via ester linkages.
The polymer itself is not on the List, but it contains phenol, 2-amino-, which is in the table of specified chemicals for genetic toxicity. So he needs to consider whether an exception applies to this chemical. If an exception does not apply, the polymer would be considered to have the same high hazard characteristics as phenol, 2-amino- (genetic toxicity).The number average molecular weight (NAMW) of Paul’s polymer is 4,500 g/mol with 17% by mass having a molecular weight less than 1,000 g/mol and 8% by mass having a molecular weight less than 500 g/mol. The exception criteria for polymers states that there must be low levels of low molecular weight species which means less than 10% (by mass) of molecules with a molecular weight that is less than 500 g/mol and less than 25% (by mass) of molecules with a molecular weight that is less than 1,000 g/mol. Therefore, the polymer meets the exception criteria and is not considered to have the genetic toxicity hazard characteristic.
Paul continues through step 4.4 of the categorisation process and next needs to follow our guidance on checking human health hazard band B hazard characteristics.
Human health hazard band B hazard characteristics
This page accompanies step 4.4 Work out your human health hazard characteristics.
Human health hazard characteristics are split into hazard bands. Hazard characteristics of most concern are in hazard band C, while those of lower concern are in hazard band A. You must always start at hazard band C. Step 4.4 tells you when you can stop working through your chemical's human health hazard characteristics and when you need to check each of them - ie C, B and A.
Hazard band B has 9 hazard characteristics you need to consider:
- High molecular weight polymer that is water absorbing
- Respiratory sensitisation
- Corrosive to the respiratory tract
- Specific target organ toxicity and a single exposure (significant toxicity)
- Skin corrosion
- Eye damage
- Skin sensitisation
- Acute toxicity (fatal or toxic)
- Specific target organ toxicity after repeated exposure
Instructions
You must always start at hazard band C.
You only need to work through the hazard characteristics on this page if your introduction is in:
- human health exposure band 3, and you are trying to get to an outcome of very low indicative human health risk or
- human health exposure band 4, and you are trying to get to an outcome of low or very low indicative human health risk.
Work your way through each hazard characteristic on this page. Look at whether your chemical meets the hazard characteristic definition based on the information that you have.
If it does meet the hazard characteristic definition, stop there - your introduction's human health hazard band is B. Move on to the next step - step 4.5 Work out your human health risk for categorisation.
If it does not meet the hazard characteristic definition, you’ll need to try and prove that your chemical does not have this hazard characteristic. The information that you need to prove this for each hazard characteristic is shown below. If you do not have this information, stop there - your introduction’s human health hazard band is B. Move onto the next step – step 4.5 Work out your human health risk for categorisation.
If you do have this information (so you can prove that the chemical does not have the hazard characteristic), move onto the next hazard characteristic on this page.
After you have considered all the hazard characteristics on this page and have proven that the chemical does not have any of them, decide whether you can stop there or continue to human health hazard band A This depends on the exposure band of your introduction.
If your introduction is in human health exposure band 3, continue to human health hazard band A.
If your introduction is in human health exposure band 4, you can choose to stop (and go to step 4.5 to work out your human health risk for categorisation), or continue to human health hazard band A.
High molecular weight polymer that is water absorbing
High molecular weight polymer that is water absorbing means that all of the following apply to the industrial chemical:
- it is a high molecular weight polymer, and
- it has a number average molecular weight that is greater than or equal to 10,000 g/mol, and
- it is capable of absorbing its own weight, or more, in water, and
- it contains particles with a particle size less than 10 micrometres (microns).
Information required to demonstrate the absence of the hazard characteristic, high molecular weight polymer that is water absorbing
If the chemical is a high molecular weight polymer, the information required to demonstrate that it does not have the hazard characteristic, high molecular weight polymer that is water absorbing, is at least one of:
- molecular weight information that demonstrates the number average molecular weight (NAMW) is less than 10,000 g/mol, or
- information that demonstrates that the polymer is not introduced in a particulate form, or
- particle size information that demonstrates that the particle size is greater than or equal to 10 micrometres (microns), or
- information that demonstrates that the polymer does not absorb its own weight or more in water, such as experiments that show that it does not form a gel in water, or that if it does, the gel dissolves upon addition of more water.
Respiratory sensitisation
Respiratory sensitisation means that any of the following apply to the industrial chemical:
- the chemical is known or presumed to produce hypersensitivity of the airways in humans, as described in chapter 3.4 of the GHS, with the chemical classified as respiratory sensitisation (category 1), or
- the chemical is named:
- on the EU SVHC Candidate list for authorisation, based on respiratory sensitising properties (https://echa.europa.eu/candidate-list-table), or
- in the Danish EPA (Q)SAR Database as a predicted respiratory sensitiser (http://qsar.food.dtu.dk/), or
- the chemical is an enzyme, or
- the chemical is a polymer that contains one or more free isocyanate groups, or
- an in vivo study on the chemical indicates hypersensitivity of the airways, as discussed in chapter 3.4 of the GHS, or
- an in vitro study on the chemical:
- indicates hypersensitivity of the airways, as discussed in chapter 3.4 of the GHS, and
- the result of the study has not been negated by in vivo studies conducted on the chemical for hypersensitivity of the airways.
Information required to demonstrate the absence of the hazard characteristic, respiratory sensitisation
There are no information requirements to demonstrate the absence of the hazard characteristic, respiratory sensitisation. If you do not have any of the information listed above that demonstrates that the chemical has this hazard characteristic then you can assume it does not for the purposes of categorisation.
Note: The information can include that the chemical is an enzyme or a polymer that contains one or more free isocyanate groups.
Corrosive to the respiratory tract
Corrosive to the respiratory tract means that any of the following apply to the industrial chemical:
- the chemical is known to cause destruction of the respiratory tract tissue, as described in chapter 3.1 of the GHS, with the chemical classified as corrosive to the respiratory tract (AUH071 - non-GHS hazard statement), or
- an in vivo study on the chemical conducted following an acceptable test guideline for acute inhalation toxicity, subacute inhalation toxicity or subchronic inhalation toxicity results in destruction of the respiratory tract, as described in chapter 3.1 of the GHS.
Information required to demonstrate the absence of the hazard characteristic, corrosive to the respiratory tract
There are no information requirements to demonstrate the absence of the hazard characteristic, corrosive to the respiratory tract. If you do not have any of the information listed above that demonstrates that the chemical has this hazard characteristic, then you can assume it does not for the purposes of categorisation.
Specific target organ toxicity after a single exposure (significant toxicity)
Specific target organ toxicity after a single exposure (significant toxicity) means that any of the following apply to the industrial chemical:
- the chemical is known or presumed to produce significant toxicity in humans, as described in chapter 3.8 of the GHS, with the chemical classified as specific target organ toxicity – single exposure (category 1), or
- an in vivo study on the chemical:
- conducted following an acceptable test guideline for acute oral toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.8 of the GHS, at less than or equal to 300 mg/kg bw, or
- conducted following an acceptable test guideline for acute dermal toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.8 of the GHS, at less than or equal to 1,000 mg/kg bw, or
- conducted following an acceptable test guideline for acute inhalation toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.8 of the GHS:
- gases - less than or equal to 2,500 ppmV/4h, or
- vapours - less than or equal to mg/L/4h, or
- for dusts/mists/fumes - less than or equal to 1 mg/L/4h.
Information required to demonstrate the absence of the hazard characteristic, specific target organ toxicity after a single exposure (significant toxicity)
There are no information requirements to demonstrate the absence of the hazard characteristic, specific target organ toxicity after a single exposure (significant toxicity). If you do not have any of the information listed above that demonstrates that the chemical has this hazard characteristic, then you can assume it does not for the purposes of categorisation.
Skin corrosion
Skin corrosion means that any of the following apply to the industrial chemical:
- the chemical is known to produce irreversible damage to the skin, as described in chapter 3.2 of the GHS, with the chemical classified as skin corrosion (category 1), or
- an in vitro study on the chemical conducted following an acceptable test guideline for skin corrosion results in the prediction of skin corrosion effects, or
- an in vivo study on the chemical conducted following an acceptable test guideline for skin irritation results in destruction of skin tissue, as described for skin corrosion in chapter 3.2 of the GHS, or
- the chemical is a pyrophoric liquid or a pyrophoric solid, as described in chapters 2.9 and 2.10 of the GHS respectively.
Information required to demonstrate the absence of the hazard characteristic, skin corrosion
The information required to demonstrate that a chemical does not have the hazard characteristic, skin corrosion, is at least one of the following:
- if the human health exposure band for the introduction is 3 or 4
- information that demonstrates that the chemical is a high molecular weight polymer that does not contain any of the following reactive functional groups:
- anhydride, or
- epoxide, or
- sulfonic acid, or
- amine, or
- information that demonstrates that the chemical is a high molecular weight polymer that contains any of the following reactive functional groups and the polymer has a combined functional group equivalent weight of greater than or equal to 1,000 g/mol:
- anhydride, or
- epoxide, or
- sulfonic acid, or
- amine, or
- test results from an in vitro study on the chemical or from suitable read across information, conducted following an acceptable test guideline for skin corrosion, with a non-corrosive prediction, or
- test results from an in vitro study on the chemical or from suitable read across information, conducted following an acceptable test guideline for skin irritation, with a non-irritant prediction, or
- test results from an in vivo study on the chemical or from suitable read across information, conducted following an acceptable test guideline for skin irritation, which does not result in destruction of skin tissue, as described for skin corrosion (category 1) in chapter 3.2 of the GHS.
- information that demonstrates that the chemical is a high molecular weight polymer that does not contain any of the following reactive functional groups:
- if the human health exposure band for the introduction is 3 – a suitable in silico prediction indicating that the chemical is not irritating to skin or has no alerting groups for skin irritation.
Eye damage
Eye damage means that any of the following apply to the industrial chemical:
- the chemical is known to produce serious eye damage, as described in chapter 3.3 of the GHS, with the chemical classified as eye damage (category 1), or
- an in vitro study on the chemical conducted following an acceptable test guideline for eye damage results in the prediction of serious eye damage effects, or
- an in vivo study on the chemical conducted following an acceptable test guideline for eye irritation results in effects on the eye, as described for serious eye damage in chapter 3.3 of the GHS, or
- the chemical is a pyrophoric liquid or a pyrophoric solid, as described in chapters 2.9 and 2.10 of the GHS, respectively.
Information required to demonstrate the absence of the hazard characteristic, eye damage
The information required to demonstrate that a chemical does not have the hazard characteristic, eye damage, is at least one of the following:
- if the human health exposure band for the introduction is 3 or 4
- information that demonstrates that the chemical is a high molecular weight polymer that does not contain any of the following reactive functional groups:
- anhydride, or
- epoxide, or
- sulfonic acid, or
- amine, or
- information that demonstrates that the chemical is a high molecular weight polymer that contains any of the following reactive functional groups with a combined functional group equivalent weight of greater than or equal to 1,000 g/mol:
- anhydride, or
- epoxide, or
- sulfonic acid, or
- amine, or
- test results from an in vitro study on the chemical or from suitable read across information, conducted following an acceptable test guideline for eye damage, which predicts the chemical would not induce serious eye damage
- test results from an in vivo study on the chemical or from suitable read across information, conducted following an acceptable test guideline for eye irritation, which does not result in effects on the eye, as described for eye damage in chapter 3.3 of the GHS.
- information that demonstrates that the chemical is a high molecular weight polymer that does not contain any of the following reactive functional groups:
- if the human health exposure band for the introduction is 3 - a suitable in silico prediction indicating that the chemical is not irritating to the eye.
Skin sensitisation
Skin sensitisation means that any of the following apply to the industrial chemical:
- the chemical is known to cause an allergic response following skin contact, as described in chapter 3.4 of the GHS, with the chemical classified as skin sensitisation (category 1), or
- human testing or epidemiological studies on the chemical result in evidence of an allergic response, as described in chapter 3.4 of the GHS, or
- an in vitro study on the chemical:
- conducted following an acceptable test guideline for skin sensitisation, results in the prediction of skin sensitisation effects, and
- the results of the study have not been negated by in vivo studies conducted on the chemical for skin sensitisation, or
- an in chemico study on the chemical:
- conducted following an acceptable test guideline for skin sensitisation, results in the prediction of skin sensitisation effects, and
- the results of the study have not been negated by in vivo studies conducted on the chemical for skin sensitisation, or
- an in vivo study on the chemical conducted following an acceptable test guideline for skin sensitisation, results in the induction of an allergic response, as described in chapter 3.4 of the GHS.
Information required to demonstrate the absence of the hazard characteristic, skin sensitisation
- There are no information requirements to demonstrate the absence of the hazard characteristic, skin sensitisation, if the chemical is corrosive to the skin (GHS category 1) as the in vivo tests cannot be conducted
- otherwise, if the human health exposure band is 3 or 4, the information required to demonstrate that a chemical does not have the hazard characteristic, skin sensitisation, is at least one of the following:
- information that demonstrates that the chemical is a high molecular weight polymer for which at least one of the following applies:
- it contains only low concern reactive functional groups, or
- it contains only low concern reactive functional groups and unsubstituted positions ortho and para to phenolic hydroxyl groups, or
- the only reactive functional groups it contains are unsubstituted positions ortho and para to phenolic hydroxyl groups, or
- it contains only low and moderate concern reactive functional groups, with a combined functional group equivalent weight for the moderate-concern functional groups of greater than or equal to 1000 g/mol, or
- it contains only moderate concern reactive functional groups, with a combined functional group equivalent weight of greater than or equal to 1000 g/mol, or
- it has a number average molecular weight that is greater than or equal to 10,000 g/mol and both:
- less than 2% by mass of molecules with molecular weight that is less than 500 g/mol, and
- less than 5% by mass of molecules with molecular weight that is less than 1,000 g/mol, or
- information that demonstrates that the chemical is a substance covered by Entry 9 of Annex V, of the REACH Regulation, or
- results from a defined approach (combination of tests) described in OECD TG 497, with a non-sensitising prediction*, or
- all of the following:
- test results from an in chemico test on the chemical or from suitable read-across information, conducted following an acceptable test guideline for the 1st key event in the adverse outcome pathway for skin sensitisation, with a non-sensitising prediction, and
- test results from an in vitro study on the chemical or from suitable read across information, conducted following an acceptable test guideline for the 2nd key event in the adverse outcome pathway for skin sensitisation, with a non-sensitising prediction, and
- test results from an in vitro study on the chemical or from suitable read across information, conducted following an acceptable test guideline for the 3rd key event in the adverse outcome pathway for skin sensitisation, with a non-sensitising prediction
- test results from an in vivo study on the chemical or from suitable read-across information, conducted following an acceptable test guideline for skin sensitisation, which does not result in induction of an allergic response, as described in chapter 3.4 of the GHS
- information that demonstrates that the chemical is a high molecular weight polymer for which at least one of the following applies:
- in addition, if the human health exposure band for the introduction is 4 and the chemical is a UV filter or is introduced for an end use in a tattoo ink, information is required to justify why the chemical would not cause skin sensitisation mediated by UV light. This may include one or more of the following :
- the chemical has a molar extinction coefficient/absorption coefficient of less than 1,000Lmol-1cm-1 at wavelengths between 290 and 700nm (based on the results of a study following OECD test guideline 101), or
- results from in vitro phototoxicity studies, or
- results from in vitro or in vivo skin sensitisation studies where the methods have been modified to include photoactivation.
Low concern reactive functional groups – see our polymer of low concern criteria page.
Moderate concern reactive functional groups – see our polymer of low concern criteria page.
*Note that negative results only with key event 1 and key event 2 adverse outcome pathway (AOP) assays will not rule out skin sensitisation potential for some weak skin sensitisers and pre-/pro-hapten fragrance chemicals when using the "2 out of 3" (2o3) defined approach and hence this should be considered when determining an appropriate testing methodology. For further information see: ENV/CBC/MONO(2021)11. Supporting Document to the OECD Guideline 497 on Defined Approaches for Skin Sensitisation
Acute toxicity (fatal or toxic)
Acute toxicity (fatal or toxic) means that any of the following apply to the industrial chemical:
- the chemical is known to exhibit acute toxicity effects, as described in chapter 3.1 of the GHS, with the chemical classified as acute toxicity (category 1 or 2 or 3), or
- an in vitro study on the chemical:
- conducted following an acceptable test guideline for acute oral toxicity, results in a predicted acute oral toxicity LD50 value of less than or equal to 300 mg/kg bw, and
- the results of the study have not been negated by in vivo studies conducted on the chemical for acute toxicity, or
- an in vivo study on the chemical:
- conducted following an acceptable test guideline for acute oral toxicity results in an acute oral LD50 value of less than or equal to 300 mg/kg bw, or
- conducted following an acceptable test guideline for acute dermal toxicity results in an acute dermal LD50 value of less than or equal to 1,000 mg/kg bw, or
- conducted following an acceptable test guideline for acute inhalation toxicity results in an acute inhalation LC50 value of:
- for gases - less than or equal to 2,500 ppmV/4h, or
- for vapours - less than or equal to 10 mg/L/4h, or
- for dusts/mists/fumes - less than equal to 1 mg/L/4h , or
- evidence in humans of systemic toxicity after eye contact, with the chemical classified with the non-GHS hazard statement – AUH070, or
- an in vivo study, conducted following an acceptable test guideline for eye irritation, results in overt signs of systemic toxicity or mortality, which is likely to be attributed to absorption of the chemical through the mucous membranes of the eye, with the chemical classified with the non-GHS hazard statement – AUH070.
Information required to demonstrate the absence of the hazard characteristic, acute toxicity (fatal or toxic)
- there are no information requirements to demonstrate the absence of the hazard characteristic, acute toxicity (fatal or toxic), if the chemical is corrosive to the skin (GHS category 1), or likely to be corrosive to the skin (that is, the chemical is a strong acid (pH less than or equal to 2.0) or base (pH greater than or equal to 11.5), and has high buffering capacity (if relevant)), as the in vivo tests cannot be conducted
- otherwise, the information required to demonstrate that a chemical does not have the hazard characteristic, acute toxicity (fatal or toxic), is at least one of the following:
- if the human health exposure band for the introduction is 3 – both of the following:
- a suitable in silico prediction for acute toxicity (LD50) of the chemical of greater than 2,000 mg/kg bw/day, and
- test results from an in vitro study on the chemical or from suitable read across information for acute toxicity (LD50), conducted following an acceptable test guideline for acute oral toxicity, of greater than 300 mg/kg bw, or
- if the human health exposure band for the introduction is 3 or 4
- information that demonstrates that the chemical is a high molecular weight polymer that has:
- less than 5% by mass of molecules with molecular weight less than 1,000 g/mol, and
- less than 2% by mass of molecules with molecular weight less than 500 g/mol, or
- information that demonstrates that the chemical is a high molecular weight polymer that has:
- inclusion of the chemical in the Select Committee on GRAS Substances (SCOGS) Database as a Type 1 conclusion, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- the chemical has been notified to the US FDA GRAS notification program and FDA had no questions about the notifier’s conclusion of GRAS status, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- the chemical is permitted to be used as a food additive according to Schedule 15 of the Australia New Zealand Food Standards Code - Standard 1.3.1 - Food Additives, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- information that demonstrates that the chemical is a substance covered by Entry 9 of Annex V of the REACH Regulation, or
- a test result from at least one in vivo study on the chemical or from suitable read across information, as detailed below, with the administration route dependent on the most relevant route of exposure (or the oral route if information on the most relevant route is not available):
- conducted following an acceptable test guideline for acute oral toxicity with an LD50 greater than 300 mg/kg bw, or
- conducted following an acceptable test guideline for acute dermal toxicity with an LD50 greater than 1,000 mg/kg bw, or
- conducted following an acceptable test guideline for acute inhalation toxicity, with an LC50:
- for gases - greater than 2,500 ppmV/4h, or
- for vapours - greater than 10 mg/L/4h, or
- for dusts/mists/fumes - greater than 1 mg/L/4h, or
- test results from an in vivo study via the oral route on the chemical or from suitable read across information, conducted following an acceptable test guideline for subacute oral toxicity, with a NOAEL greater than or equal to 1,000 mg/kg bw/day.
- if the human health exposure band for the introduction is 3 – both of the following:
Specific target organ toxicity after repeated exposure
Specific target organ toxicity after repeated exposure means that any of the following apply to the industrial chemical:
- the chemical is known to exhibit significant toxicity or be potentially harmful to human health following repeated exposure, as described in chapter 3.9 of the GHS, with the chemical classified as specific target organ toxicity – repeated exposure (category 1 or 2), or
- an in vivo study on the chemical:
- conducted following an acceptable test guideline for subacute oral toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.9 of the GHS, and a NOAEL (oral) value of less than 300 mg/kg bw/day, or
- conducted following an acceptable test guideline for subchronic oral toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.9 of the GHS, and a NOAEL (oral) of less than 100 mg/kg bw/day, or
- conducted following an acceptable test guideline for subacute dermal toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.9 of the GHS, and a NOAEL (dermal) value of less than 600 mg/kg bw/day, or
- conducted following an acceptable test guideline for subchronic dermal toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.9 of the GHS, and a NOAEL (dermal) of less than 200 mg/kg bw/day, or
- conducted following an acceptable test guideline for subacute inhalation toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.9 of the GHS, and a NOAEC (inhalation) of:
- for gases - less than 750 ppmV/6 h/day, or
- for vapours - less than 3 mg/L/6 h/day, or
- for dusts/mists/fumes less than 0.6 mg/L/6 h/day, or
- conducted following an acceptable test guideline for subchronic inhalation toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.9 of the GHS, and a NOAEC (inhalation) of:
- for gases - less than 250 ppmV/6 h/day, or
- for vapours - less than 1 mg/L/6 h/day, or
- for dusts/mists/fumes less than 0.2 mg/L/6 h/day.
Information required to demonstrate the absence of the hazard characteristic, specific target organ toxicity after repeated exposure
Information is required to demonstrate the absence of the hazard characteristic, specific target organ toxicity after repeated exposure, if:
- the human health exposure band for the introduction is 4, and
- you are seeking to demonstrate that the introduction meets the criteria for low risk or very low risk
and any of the following apply:
- the human health categorisation volume for the introduction is greater than 100 kg and the chemical is introduced for end use in any of the following types of articles:
- food contact, or
- children’s toys that can be placed in the mouth, or
- children’s care products that can be placed in the mouth, or
- the human health categorisation volume for the introduction is greater than 1000 kg and the chemical is not introduced for end use in any of the following types of articles:
- food contact, or
- children’s toys that can be placed in the mouth, or
- children’s care products that can be placed in the mouth.
See extra guidance on categorisation of chemicals:
If 1 or 2 apply, the information required to demonstrate that a chemical does not have the hazard characteristic, specific target organ toxicity after repeated exposure, is at least one of the following:
- inclusion of the chemical in the Select Committee on GRAS Substances (SCOGS) Database as a Type 1 conclusion, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- the chemical has been notified to the US FDA GRAS notification program and FDA had no questions about the notifier’s conclusion of GRAS status, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- the chemical is permitted to be used as a food additive according to Schedule 15 of the Australia New Zealand Food Standards Code - Standard 1.3.1 - Food Additives, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- information that demonstrates that the chemical is a substance covered by Entry 9 of Annex V of the REACH Regulation, or
- information that demonstrates that the chemical is a high molecular weight polymer that has
- < 5% by mass of molecules with molecular weight < 1000 g/mol, and
- < 2% by mass of molecules with molecular weight < 500 g/mol, or
- a test result from at least one in vivo study on the chemical or from suitable read across information, as detailed below, with the administration route dependent on the most relevant route of exposure (or the oral route if information on the most relevant route is not available):
- conducted following an acceptable test guideline for subacute oral toxicity, in which the NOAEL (oral) is greater than or equal to 300 mg/kg bw/day or there were no significant toxic effects of relevance to human health (as described in chapter 3.9 of the GHS) produced, or
- conducted following an acceptable test guideline for subchronic oral toxicity, in which the NOAEL (oral) is greater than or equal to 100 mg/kg bw/day or there were no significant toxic effects of relevance to human health (as described in chapter 3.9 of the GHS) produced, or
- conducted following an acceptable test guideline for subacute dermal toxicity, in which the NOAEL (dermal) is greater than or equal to 600 mg/kg bw/day or there were no significant toxic effects of relevance to human health (as described in chapter 3.9 of the GHS) produced, or
- conducted following an acceptable test guideline for subchronic dermal toxicity, in which the NOAEL (dermal) is greater than or equal to 200 mg/kg bw/day or there were no significant toxic effects of relevance to human health (as described in chapter 3.9 of the GHS) produced, or
- conducted following an acceptable test guideline for subacute inhalation toxicity, in which there were no significant toxic effects of relevance to human health (as described in chapter 3.9 of the GHS) produced, or the NOAEC (inhalation) is:
- for gases - greater than or equal to 750 ppmV/6 h/day, or
- for vapours - greater than or equal to 3 mg/L/6 h/day, or
- for dusts/mists/fumes - greater than or equal to 0.6 mg/L/6 h/day , or
- conducted following an acceptable test guideline for subchronic inhalation toxicity, in which there were no significant toxic effects of relevance to human health (as described in chapter 3.9 of the GHS) produced, or the NOAEC (inhalation) is:
- for gases - greater than or equal to 250 ppmV/6 h/day, or
- for vapours - greater than or equal to 1 mg/L/6 h/day, or
- for dusts/mists/fumes - greater than or equal to 0.2 mg/L/6 h/day.
There are no information requirements to demonstrate the absence of the hazard characteristic, specific target organ toxicity after repeated exposure, if:
- the human health exposure band for the introduction is 4, and
- you are seeking to demonstrate that the introduction meets the criteria for low risk or very low risk,
and any of the following apply:
- the human health categorisation volume for the introduction is less than or equal to 100 kg and the chemical is introduced for end use in any of the following types of articles:
- food contact, or
- children’s toys that can be placed in the mouth, or
- children’s care products that can be placed in the mouth, or
- the human health categorisation volume for the introduction is less than or equal to 1,000 kg and the chemical is not introduced for end use in any of the following types of articles:
- food contact, or
- children’s toys that can be placed in the mouth, or
- children’s care products that can be placed in the mouth
In these circumstances, if you do not have any information that demonstrates that the chemical has this hazard characteristic then you can assume it does not for the purposes of categorisation.
Human health hazard band A hazard characteristics
Human health hazard characteristics are split into hazard bands. Hazard characteristics of most concern are in hazard band C, while those of lower concern are in hazard band A.
Hazard band A has 6 hazard characteristics you need to consider:
- High molecular weight polymer that has lung overloading potential
- Aspiration hazard
- Specific target organ toxicity after a single exposure (harmful or transient effects)
- Skin irritation
- Eye irritation
- Acute toxicity (harmful)
Instructions
You must always start at hazard band C. You only need to work through the hazard characteristics on this page if your introduction is in:
- human health exposure band 3, and you are trying to get to an outcome of very low indicative human health risk or
- human health exposure band 4, and you are trying to get to an outcome of very low indicative human health risk.
Work your way through each hazard characteristic on this page. Look at whether your chemical meets the hazard characteristic definition based on the information that you have.
If it does meet the hazard characteristic definition, stop there - your introduction's human health hazard band is A.
Move on to the next step - step 4.5 Work out your human health risk for categorisation.
If it does not meet the hazard characteristic definition, you’ll need to try and prove that your chemical does not have this hazard characteristic. The information that you need to prove this for each hazard characteristic is shown below. If you do not have this information, stop there - your introduction’s human health hazard band is A.
Move onto the next step – step 4.5 Work out your human health risk for categorisation.
If you do have this information (so you can prove that the chemical does not have the hazard characteristic), move onto the next hazard characteristic on this page.
After you have considered all the hazard characteristics on this page and have proven that the chemical does not have any of them, go to step 4.5 to work out your human health risk for categorisation).
High molecular weight polymer that has lung overloading potential
This page accompanies step 4.4 Work out your human health hazard characteristics.
High molecular weight polymer that has lung overloading potential means that all of the following apply to the industrial chemical:
- it is a polymer, and
- it has a number average molecular weight that is greater than 70,000 g/mol, and
- it has a solubility in water of less than 0.1 mg/L, and
- it becomes aerosolised during end use.
Information required to demonstrate the absence of the hazard characteristic, high molecular weight polymer that has lung overloading potential
If the chemical is a polymer, the information required to demonstrate that a chemical does not have the hazard characteristic, high molecular weight polymer that has lung overloading potential, is at least one of the following:
- molecular weight information that demonstrates that the number average molecular weight is less than or equal to 70,000 g/mol, or
- information that demonstrates that the polymer has a solubility in water that is greater than or equal to 0.1 mg/L measured following an acceptable test guideline for water solubility, or
- information that demonstrates that the polymer does not become aerosolised during end use
Aspiration hazard
Aspiration hazard means that any of the following apply to the industrial chemical:
- the chemical is known or presumed to cause aspiration toxicity, as described in chapter 3.10 of the GHS, with the chemical classified as may be fatal if swallowed and enters airways (category 1), or
- the chemical is a hydrocarbon that has a kinematic viscosity less than or equal to 20.5 mm2/s, measured at 40°C.
Information required to demonstrate the absence of the hazard characteristic, aspiration hazard
- If the chemical is a hydrocarbon, the information required to demonstrate the absence of the hazard characteristic, aspiration hazard, is a measured kinematic viscosity greater than a 20.5mm2/s, at 40 °C
- Otherwise, there are no information requirements to demonstrate the absence of the hazard characteristic, aspiration hazard. If you do not have any information that demonstrates that the chemical has this hazard characteristic, then you can assume it does not for the purposes of categorisation.
Specific target organ toxicity after a single exposure (harmful or transient effects)
Specific target organ toxicity after a single exposure (harmful or transient effects) means that any of the following apply to the industrial chemical:
- the chemical is known or presumed to be harmful to humans or to cause transient target organ effects, as described in chapter 3.8 of the GHS, with the chemical classified as specific target organ toxicity-single exposure (category 2 or 3), or
- an in vivo study on the chemical:
- conducted following an acceptable test guideline for acute oral toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.8 of the GHS, at greater than 300 but less than or equal to 2,000 mg/kg bw, or
- conducted following an acceptable test guideline for acute dermal toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.8 of the GHS, at greater than 1,000 but less than or equal to 2,000 mg/kg bw, or
- conducted following an acceptable test guideline for acute inhalation toxicity results in significant toxic effects of relevance to human health, as discussed in chapter 3.8 of the GHS, at:
- for gases - greater than 2,500 but less than or equal to 20,000 ppmV/4h, or
- for vapours - greater than 10 but less than or equal to 20 mg/L/4h, or
- for dusts/mists/fumes - greater than 1 but less than or equal to 5 mg/L/4h.
Information required to demonstrate the absence of the hazard characteristic, specific target organ toxicity after a single exposure (harmful or transient effects)
There are no information requirements to demonstrate the absence of the hazard characteristic, specific target organ toxicity after a single exposure (harmful or transient effects). If you do not have any information that demonstrates that the chemical has this hazard characteristic, then you can assume it does not for the purposes of categorisation.
Skin irritation
Skin irritation means that any of the following apply to the industrial chemical:
- the chemical is known to produce reversible damage to the skin, as described in chapter 3.2 of the GHS, with the chemical classified as skin irritation (category 2), or
- an in vitro study on the chemical conducted following an acceptable test guideline for skin irritation results in the prediction of skin irritation effects, or
- an in vivo study on the chemical conducted following an acceptable test guideline for skin irritation results in skin reactions, as described for skin irritation (category 2) in chapter 3.2 of the GHS.
Information required to demonstrate the absence of the hazard characteristic, skin irritation
The information required to demonstrate that a chemical does not have the hazard characteristic, skin irritation, is at least one of the following:
- if the human health exposure band for the introduction is 3 - a suitable in silico prediction indicating that the chemical is not irritating to the skin, or
- test results from an in vitro study on the chemical or from suitable read across information, conducted following an acceptable test guideline for skin irritation, with a non-irritant prediction, or
- if the human health exposure band for the introduction is 3 or 4
- test results from an in vitro study on the chemical or from suitable read across information, conducted following an acceptable test guideline for skin irritation, with a non-irritant prediction, or
- test results from an in vivo study on the chemical or from suitable read across information, conducted following an acceptable test guideline for skin irritation, which does not result in skin reactions, as described for skin irritation (category 2) in chapter 3.2 of the GHS, or
- test results from an in vivo study on the chemical or from suitable read across information conducted following an acceptable test guideline for acute dermal toxicity, that when tested at 2,000 mg/kg bw/day is not irritating to the skin.
In addition, if the chemical is introduced for an end use in a tattoo ink, information is required to justify why the chemical would not cause skin irritation mediated by UV light. This may include one or more of the following:
- the chemical has a molar extinction coefficient/absorption coefficient of less than 1,000Lmol-1cm-1 at wavelengths between 290 and 700 nm (based on the results of a study following OECD test guideline 101), or
- results from in vitro phototoxicity studies, or
- results from in vitro or in vivo irritation studies where the methods have been modified to include photoactivation.
Eye irritation
Eye irritation means that any of the following apply to the industrial chemical:
- the chemical is known to produce changes in the eye, as described in chapter 3.3 of the GHS, with the chemical classified as eye irritation (category 2), or
- an in vitro study on the chemical conducted following an acceptable test guideline for eye irritation results in the prediction of eye irritation effects, or
- an in vivo study on the chemical conducted following an acceptable test guideline for eye irritation results in changes in the eye, as described for eye irritation in chapter 3.3 of the GHS.
Information required to demonstrate the absence of the hazard characteristic, eye irritation
The information required to demonstrate that a chemical does not have the hazard characteristic, eye irritation, is at least one of the following:
- if the human health exposure band for the introduction is 3 - a suitable in silico prediction indicating that the chemical is not irritating to the eyes, or
- if the human health exposure band for the introduction is 3 or 4
- test results from an in vitro study on the chemical or from suitable read across information, conducted following an acceptable test guideline for eye damage or eye irritation with a non-irritant prediction, or
- test results from an in vivo study on the chemical or from suitable read across information, conducted following an acceptable test guideline for eye irritation, which does not result in changes in the eyes, as described for eye irritation (category 2) in chapter 3.3 of the GHS.
Acute toxicity (harmful)
Acute toxicity (harmful) means that any of the following apply to the industrial chemical:
- the chemical is known to exhibit acute toxicity effects, as described in chapter 3.1 of the GHS, with the chemical classified as acute toxicity (category 4), or
- an in vitro study on the chemical:
- conducted following an acceptable test guideline for acute oral toxicity, results in a predicted acute toxicity LD50 value of greater than 300 but less than or equal to 2,000 mg/kg bw, and
- the results of the study have not been negated by in vivo studies conducted on the chemical for acute toxicity, or
- an in vivo study on the chemical:
- conducted following an acceptable test guideline for acute oral toxicity results in an acute oral LD50 value of greater than 300 but less than or equal to 2,000 mg/kg bw, or
- conducted following an acceptable test guideline for acute dermal toxicity results in an acute dermal LD50 value of greater than 1,000 but less than or equal to 2,000 mg/kg bw, or
- conducted following an acceptable test guideline for acute inhalation toxicity results in an acute inhalation LC50 value of:
- for gases - greater than 2500 but less than or equal to 20,000 ppmV/4h, or
- for vapours - greater than 10 but less than or equal to 20 mg/L/4h, or
- for dusts/mists/fumes - greater than 1 but less than or equal to 5 mg/L/4h.
Information required to demonstrate the absence of the hazard characteristic, acute toxicity (harmful)
- the information required to demonstrate that a chemical does not have the hazard characteristic, acute toxicity (harmful), is at least one of the following:
- if the human health exposure band for the introduction is 3 - both of the following:
- a suitable in silico prediction for acute toxicity (LD50) of the chemical of greater than 2,000 mg/kg bw/day, and
- test results from an in vitro study on the chemical or from suitable read across information, conducted following an acceptable test guideline for acute oral toxicity, with a predicted LD50 of greater than 2,000 mg/kg bw, or
- if the human health exposure band for the introduction is 3 or 4
- information that demonstrates that the chemical is a high molecular weight polymer that has:
- less than 5% by mass of molecules with molecular weight less than 1,000 g/mol, and
- less than 2% by mass of molecules with molecular weight less than 500 g/mol
- inclusion of the chemical in the Select Committee on GRAS Substances (SCOGS) Database as a Type 1 conclusion, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- the chemical has been notified to the US FDA GRAS notification program and FDA had no questions about the notifier’s conclusion of GRAS status, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- the chemical is permitted to be used as a food additive according to Schedule 15 of the Australia New Zealand Food Standards Code - Standard 1.3.1 - Food Additives, as long as the human health exposure expected from the industrial use of the chemical is no higher than the human health exposure expected from food use, or
- information that demonstrates that the chemical is a substance covered by Entry 9 of Annex V of the REACH Regulation, or
- a test result from at least one in vivo study on the chemical or from suitable read across information, as detailed below, with the administration route dependent on the most relevant route of exposure (or the oral route if information on the most relevant route is not available):
- conducted following an acceptable test guideline for acute oral toxicity with an LD50 greater than 2,000 mg/kg bw, or
- conducted following an acceptable test guideline for acute dermal toxicity with an LD50 greater than 2,000 mg/kg bw, or
- conducted following an acceptable test guideline for acute inhalation toxicity with an LC50:
- for gases - greater than 20,000 ppmV/4h, or
- for vapours - greater than 20 mg/L/4h, or
- for dusts/mists/fumes - greater than 5 mg/L/4h, or
- test results from an in vivo study on the chemical or from suitable read across information, conducted following an acceptable test guideline for subacute oral toxicity, with a NOAEL greater than or equal to 1,000 mg/kg bw/day.
- information that demonstrates that the chemical is a high molecular weight polymer that has:
- if the human health exposure band for the introduction is 3 - both of the following:
Step 4.5 Outcome - your human health risk for categorisation
Get help with this step — explore our categorisation decision tools
We explain the table in detail for each human health exposure band that your introduction could be in. This includes what your indicative human health risk outcome will be, depending on which hazard characteristics your chemical does or does not have. Your outcome will be that your introduction has an indicative human health risk of:
- medium to high
- low OR
- very low
Refer back to step 4.4 for information about how to consider the hazard characteristics and where to start and stop when considering hazard characteristics.
Human health risk table
| Work out your indicative human health risk | Human health exposure band | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Human health hazard band | C | Low risk | Medium to high risk | Medium to high risk | Medium to high risk |
| B | Very low risk | Very low risk | Low risk | Medium to high risk | |
| A | Very low risk | Very low risk | Low risk | Low risk | |
| Not C, B, A | Very low risk | Very low risk | Very low risk | Very low risk | |
If your introduction is in human health exposure band 1
If your introduction is in human health exposure band 1, you will need to consider if your chemical has any of the hazard characteristics in human health hazard band C. It’s not necessary to consider the hazard characteristics in band B or A.
The indicative human health risk of your introduction will be:
- low if your chemical has 1 or more of the hazard characteristics in human health hazard band C or
- very low if your chemical does not have any of the hazard characteristics in human health hazard band C
The table on this page shows how you can work out your indicative human health risk by using your human health exposure band and the human health hazard characteristics that your chemical does or does not have.
If your introduction is in human health exposure band 2
If your introduction is in human health exposure band 2, you will need to consider if your chemical has any of the hazard characteristics in human health hazard band C. It’s not necessary to consider the hazard characteristics in band B or A.
The indicative human health risk of your introduction will be:
- medium to high if your chemical has 1 or more of the hazard characteristics in human health hazard band C OR
- very low if your chemical does not have any of the hazard characteristics in human health hazard band C
If your introduction is in human health exposure band 3
If your introduction is in human health exposure band 3, at a minimum, you will need to consider if your chemical has any of the hazard characteristics in human health hazard band C.
The indicative human health risk of your introduction will be:
- medium to high if your chemical has 1 or more of the hazard characteristics in human health hazard band C OR
- low if your chemical does not have any of the hazard characteristics in human health hazard band C
You can choose to stop if you get to low indicative human health risk.
If you want to see if your introduction could have a very low indicative human health risk, you will also need to consider if it has any of the hazard characteristics in human health hazard bands B and A.
The indicative human health risk of your introduction will be:
- low if your chemical has 1 or more of the hazard characteristics in human health hazard band B or A or
- very low if your chemical does not have any of the hazard characteristics in human health hazard band B or A
If your introduction is in human health exposure band 4
If your introduction is in human health exposure band 4, you will first need to consider if your chemical has any of the hazard characteristics in human health hazard band C. If it does not, then continue on to consider the hazard characteristics in human health hazard band B.
The indicative human health risk of your introduction will be:
- medium to high if your chemical has 1 or more of the hazard characteristics in human health hazard band C or B or
- low if your chemical does not have any of the hazard characteristics in human health hazard band C or B
You can choose to stop if you get to low indicative human health risk.
If you want to see if your introduction could have a very low indicative human health risk, you will also need to consider if it has any of the hazard characteristics in human health hazard band A.
The indicative human health risk of your introduction will be:
- low if your chemical has 1 or more of the hazard characteristics in human health hazard band A or
- very low if your chemical does not have any of the hazard characteristics in human health hazard band A
'Special cases' — introductions that cannot have a very low indicative human health risk
Your introduction cannot have a very low indicative human health risk if it is a:
- UV filter or
- chemical that is introduced as a solid or a dispersion that is not soluble, that meets the nanoscale particle size criteria, and the introduction of the nanoscale portion of the chemical (the part that has a particle size range of 1 nm to 100 nm) is incidental to the introduction of the non-nanoscale portion or
- chemical that is introduced as a solid or a dispersion where there is no information available on its water solubility or its particle size, and the introduction of any nanoscale portion of the chemical (the part that has a particle size range of 1 nm to 100 nm) is incidental to the introduction of the non-nanoscale portion.
If your introduction is 1 of these, and you got a very low risk outcome in this step, you need to change that outcome to low risk.
This means if your consideration of step 4.5 got you to an outcome of very low risk, your final outcome needs to be changed to low risk.
Definitions of these 'special cases'
A UV filter is a chemical that is intended to protect the skin against ultraviolet radiation in the range of 290 nm to 400 nm by absorption, reflection, or scattering of ultraviolet radiation.
Nanoscale particle size criteria means that the chemical consists of solid particles in an unbound state or as an aggregate or agglomerate. At least 50% (by number size distribution) of the particles must have at least 1 external dimension in the particle size range of 1nm to 100nm (i.e. the nanoscale).
Not soluble means the solubility of the chemical in water is less than 33.3 g/L measured following an acceptable test guideline for water solubility; or the dissolution rate of the chemical is not more than 70%.
Next
Go to step 5 to work out the risk to the environment of your introduction
Step 5: Work out your introduction's risk to the environment
To be able to finish your categorisation you need to work out the risks of your introduction to the environment. To work this out start at 5.1 and continue as far as you need to through each step.
Once you have your answer for the risk of your introduction to the environment – medium to high, low or very low – go to Step 6. In Step 6 you'll combine the human health risk and the environment risk for the final category of your introduction.
By now you know the human health risk of your introduction – medium to high, low or very low – which you completed in step 4.
Step 5.1: Introductions that are always medium to high risk for the environment
Some introductions are always medium to high risk to the environment. This means they will be in the assessed introduction category and you need to apply for an assessment certificate.
Instructions
Go through A, B, C, D and E to work out if you are, or are not, introducing any of these types of chemicals. You must keep records of study reports and other information that you used to answer each question.
A. Is your chemical a certain gas?
B. Is your chemical a certain organotin chemical?.
*D. Is your chemical a certain polyhalogenated organic chemical?.
*E. Is your chemical a certain chemical at the nanoscale?.
*Note, these last 3 types of chemical introductions we describe on this page are the same as the ones that we describe in step 4.1 for human health. This means that they are medium to high indicative risk to the environment and to human health. So, if you are introducing one of these types of chemicals, you should have already worked out that your introduction category is assessed because of its indicative human health risk being medium to high. Also, you now know that it’s also assessed because of its indicative environment risk being medium to high.
A. Is your chemical a certain gas?
Your chemical is a gas if it is in the gaseous phase at 20 oC and 101.3kPa (ambient conditions).
All introductions of chemicals that are a gas and are persistent in the environment are a specified class of introduction.
No I am not introducing this type of chemical
You must be able prove this. For example, you might have a SDS or product information sheet that indicates the appearance. You also need to be able to provide the information if we ask for it.
Next step: Go to 'B. Is your chemical a certain organotin chemical?'
Yes I am introducing this type of chemical
If you are introducing a gas, you must consider which of the following circumstances apply to your introduction.
1. Introduced at volumes less than 100kg
Next step: Go to 'B. Is your chemical a certain organotin chemical?'
2. Introduced at volumes higher than 100kg each year
You need to have information about the persistence of your gas. To prove that your gas is not persistent, we’ll accept information that shows your gas has a half-life in air of less than 2 days. This could be:
- an in silico prediction using EPI Suite AOPWIN or
- studies that use methods that are well established in published peer-reviewed scientific literature
Next step: If you do have the in silico predictions or studies to prove that your gas is not persistent, go to 'B. Is your chemical a certain organotin chemical?'.
or
If you do not have the required in silico predictions or studies described above, then you cannot prove that your gas is not persistent.
Outcome: Your introduction is medium to high indicative risk to the environment. This means your introduction is in the assessed category and called an 'assessed introduction'.
- Before you can introduce the chemical, you must apply for an assessment certificate and select 'Environment focus' as the application type or apply for a commercial evaluation authorisation (if you meet the strict criteria).
- When you apply for an assessment certificate, you need to answer ‘yes’ when we ask if your introduction is a specified class of introduction. When we receive your application, we’ll contact you to ask for extra information that we need to assess the risks of your introduction.
B. Is your chemical an organotin chemical?
Organotin chemicals are chemicals that contain at least 1 tin atom that is covalently bound to at least one carbon atom. They are widely used as polyvinyl chloride (PVC) stabilisers, biocides, and in antifouling paints.
No I am not introducing this type of chemical
You must be able prove this. You (or the chemical identity holder) need information about the identity of the chemical as proof you are not introducing this type of chemical. You also need to be able to provide the information if we ask for it.
Next step: Go to 'C. Designated fluorinated chemicals (including per- and polyfluoroalkyl substances, known as PFAS)'.
Yes I am introducing this type of chemical
If you are introducing an organotin chemical, you must consider which of the below circumstances apply to your introduction.
1. Introduced at volumes less than or equal to 10 kg per year
Next step: Go to 'C. Is your chemical a designated fluorinated carbon (including per- and polyfluoroalkyl substances, known as PFAS)?'
2. Introduced at volumes greater than 10 kg per year
Outcome: If this applies to your introduction, it is in the assessed introduction category and is called an 'assessed introduction'. Before you can introduce the chemical, you must apply for an assessment certificate and select 'Environment' focus as the application type or apply for a commercial evaluation authorisation (if you meet the strict criteria).
C. Is your chemical a designated fluorinated chemical (including per- and polyfluoroalkyl substances, known as PFAS)?
Fluorinated chemicals contain fluorine atoms and include per- and polyfluoroalkyl substances (PFAS). These are commonly used in products to add resistance to heat, other chemicals, and abrasion. They also act as dispersion, wetting or surface treatment agents. We have an increased level of concern for introductions of designated fluorinated chemicals (including PFAS) because these chemicals, or their degradation products, may be persistent in the environment, bioaccumulate and be highly toxic.
No I am not introducing this type of chemical
You must have information about your chemical's identity as proof that you're not introducing this type of chemical. You (or the chemical identity holder) need to provide the information if we ask for it.
Next step: Go to 'D. Is your chemical a certain polyhalogenated organic chemical?' below.
Yes I am introducing this type of chemical
Outcome: Your introduction has a medium to high indicative risk to both human health and the environment. This means your introduction is in the assessed category and called an 'assessed introduction'.
- Before you can introduce the chemical, you must apply for an assessment certificate and select 'Health and environment focus' as the application type or apply for a commercial evaluation authorisation (if you meet the strict criteria).
- When you apply for an assessment certificate, you need to answer ‘yes’ when we ask if your introduction is a specified class of introduction. When we receive your application, we’ll contact you to ask for extra information that we need to assess the risks of your introduction.
D. Is your chemical a polyhalogenated organic chemical?
Polyhalogenated organic chemicals are carbon-based chemicals that contain more than 1 covalently bonded halogen atom, such as bromine, chlorine, fluorine, or iodine. Polyhalogenated organic chemicals are commonly used as flame retardants in plastics, textiles, and electronic circuitry. They may have long-term effects on human health and the environment. We have an increased level of concern for introductions of chemicals that are polyhalogenated organic chemicals because these chemicals, or their degradation products, may be persistent in the environment, bioaccumulate and be highly toxic.
No I am not introducing this type of chemical
You must have information about your chemical's identity as proof that you're not introducing this type of chemical. You (or the chemical identity holder) need to provide the information if we ask for it.
Next step: Go to 'E. Is your chemical a certain chemical at the nanoscale?' below.
Yes I am introducing this type of chemical
All introductions of polyhalogenated chemicals are a specified class of introduction.
If the chemical identity information that you (or the chemical identity holder) have confirms you are introducing this type of chemical, you must consider which of the following circumstances apply to your introduction.
1. Polyhalogenated organic chemicals introduced at volumes less than or equal to 100 kg each year
Next step: Go to 'E. Is your chemical a certain chemical at the nanoscale?' below.
2. Polyhalogenated organic chemicals introduced at volumes higher than 100 kg each year
You need to have test results about the persistence of your chemical and any of its known environmental degradation products.
- Known environmental degradation products refer to the expected breakdown products of the chemical under environmentally relevant conditions. These breakdown products are ones that have been found in studies or reported in scientific literature.
- A persistent chemical remains intact in the environment for long periods of time. A chemical is persistent if its degradation half-life (T1/2) is greater than or equal to:
- 2 days in air or
- 2 months in water or
- 6 months in soil or
- 6 months in sediment.
To prove that your chemical and any of its known environmental degradation products are not persistent, we accept study results in option 1 or 2.
Option 1
A study conducted following an acceptable test guideline for ready biodegradability that results in the pass levels being reached within one of the following time periods:
- specified time period – such that the chemical is considered to be readily biodegradable or
- duration of the test – but not within the specified time period for the chemical to be considered readily biodegradable, provided biodegradation has started within the specified time period
If you have this study showing these results, then move on to 'E. Is your chemical a certain chemical at the nanoscale?' below.
Option 2
A study conducted following an acceptable test guideline for Aerobic and Anaerobic Transformation in Aquatic Sediment Systems that results in both a degradation half-life of less than 2 months in water and 6 months in sediment.
If you have this study showing these results, then move on to 'E. Is your chemical a certain chemical at the nanoscale?' below.
If you do not have either of the study results described in option 1 or 2
Outcome: Your introduction is medium to high indicative risk to human health and the environment because you cannot prove that your chemical (and any of its known environmental degradation products) are not persistent. Your introduction is medium to high indicative risk to human health and the environment. This means your introduction is in the assessed category and called an 'assessed introduction'.
- Before you can introduce the chemical, you must apply for an assessment certificate and select 'Health and environment focus' as the application type or apply for a commercial evaluation authorisation (if you meet the strict criteria).
- When you apply for an assessment certificate, you need to answer ‘yes’ when we ask if your introduction is a specified class of introduction. When we receive your application, we’ll contact you to ask for extra information that we need to assess the risks of your introduction.
E. Is your chemical a certain chemical at the nanoscale?
Introductions of chemicals that meet all 4 criteria below are medium to high indicative risk to both human health and the environment. We refer to these introductions as 'certain chemicals at the nanoscale'. We have an increased level of concern for chemicals at the nanoscale, because of uncertainty about the risks of some of these chemicals due to their potentially different properties, such as chemical reactivity, relative to the non-nanoscale forms of the chemicals.
- It is introduced as a solid or is in a dispersion.
- It consists of solid particles in an unbound state or as an aggregate or agglomerate. At least 50% (by number size distribution) of the particles have at least one external dimension in the particle size range of 1 nm to 100 nm (ie. the nanoscale). Note that if you meet criteria 1 and 2, and regardless of whether you meet criteria 3 and 4, your introduction is a specified class of introduction.
- It is not soluble. This means the solubility of the chemical in water is less than 33.3 g/L measured following an acceptable test guideline for water solubility; or the dissolution rate of the chemical is not more than 70%.
- The introduction of the nanoscale portion of the chemical (the part that has a particle size range of 1 nm to 100 nm) is not incidental to the introduction of the non-nanoscale portion. This is the case if any of the following apply:
- the manufacture of the chemical (in Australia or overseas) at the nanoscale is the result of a deliberate manufacturing decision
- the manufacture of the chemical (in Australia or overseas) at the nanoscale is necessary for the manufacture of the non-nanoscale portion of the chemical. This means that to make the non-nanoscale chemical, part of the chemical has to be at the nanoscale
- the chemical at the nanoscale has specific technical characteristics that are the intended result of changes in the manufacturing process. For example, if the process of manufacturing the chemical changes in order to change the particle size of the chemical, or its properties at the nanoscale. This could happen by:
- mechanical actions like milling, grinding, shearing, sieving or sonication
- chemicals reactions like electrochemical exfoliation, or catalysts
- other changes such as changes to pressure or temperature or pH or solvent
Yes I am introducing this type of chemical
This means that your introduction meets all 4 criteria above and is a 'certain chemical at the nanoscale'.
Outcome: Your introduction has a medium to high indicative risk to both human health and the environment. This means your introduction is in the assessed category and called an ‘assessed introduction’.
- Before you can introduce the chemical, you must apply for an assessment certificate and select 'Health and environment focus' as the application type or apply for a commercial evaluation authorisation (if you meet the strict criteria).
- When you apply for an assessment certificate, you need to answer ‘yes’ when we ask if your introduction is a specified class of introduction. When we receive your application, we’ll contact you to ask for extra information that we need to assess the risks of your introduction.
No I am not introducing this type of chemical
This means that you have information or studies to prove that your chemical does not meet any of the 4 criteria, or it only meets some of the 4 criteria. Answering the questions below will help you prove this. As you go through the questions, we'll tell you the next steps you should take.
Step 5.2 Introductions that can be low risk for the environment
This step relates to introductions that are internationally assessed for the environment. These must meet all of the following criteria to be considered ‘low indicative risk’ for the environment.
Skip this step if you are not using an internationally assessed chemical.
Note: Your introduction might still be low indicative risk for the environment but you will need to complete steps 5.3, 5.4 and 5.5 to work this out.
Step 5.2.1
Refer to our Guide to categorising internationally assessed introductions. It has extra information for introducers using international assessments and covers scenarios and outcomes for chemicals that are internationally assessed for:
- human health only
- the environment only
- both human health and the environment
It also lists the trusted overseas bodies we accept assessments from.
The relevant section to refer to in the guide to help you complete step 5.2 is Internationally assessed for the environment only.
Step 5.2.2
Once you’ve read the guide to categorising internationally assessed introductions, you'll be able to work out whether your introduction either:
- meets our criteria for internationally assessed for the environment
- does not meet our criteria for internationally assessed for the environment
Your introduction meets our criteria for internationally assessed for the environment
Option 1
- Keep the outcome you already have — your introduction is low risk for the environment; and
- Go to step 6 to complete your categorisation.
Option 2
Check to see if your introduction can be very low risk for the environment by completing the rest of step 5:
- Complete Step 5.3 — Work out your introduction's environment exposure band; then
- Complete Step 5.4 — Work out your introduction's environment hazard characteristics
Once you’ve done this, go to step 6 to complete your categorisation.
Your introduction does not meet our criteria for internationally assessed for the environment
Continue with step 5 to work out your introduction's risk for the environment.
Next:
- Complete Step 5.3 — Work out your introduction's environment exposure band; then
- Complete Step 5.4 — Work out your introduction's environment hazard characteristics
Once you’ve done this, go to step 6 to complete your categorisation.
If you've established your introduction is not medium to high risk for the environment (Step 5.1), now see if your introduction can be low risk for the environment.
Step 5.3 Work out your environment exposure band
Why you need to work out your introduction's exposure band
It is part of the process to identify the indicative environment risk of your introduction. In step 4, you also had to work out your introduction's human health exposure band.
What does an environment exposure band identify about your introduction?
It identifies the likelihood and extent of environmental exposure to the chemical. This likelihood and extent of exposure increases with each band. Exposure band 1 is the lowest exposure band, and exposure band 4 the highest. Introductions in environment exposure band 1 will have the lowest level of environmental exposure, and exposure band 4, the highest.
Information used to assign a chemical to its correct exposure band
Environment categorisation volume
Use this guidance to help you calculate this.
If your chemical has a designated kind of release into the environment
We define 'designated kind of release into the environment' (which we refer to throughout this page) to be where the chemical is intentionally released during use to land, biota, natural waterways, municipal water supplies or air (unless its only for domestic or personal use, or end use in an air freshener). It also includes any releases to the environment from firefighting end uses and releases into the ocean.
See also:
- Categorisation of chemicals intentionally released to the environment during use
- Categorisation of chemicals with an end use in firefighting
- Categorisation of chemicals with an end use offshore
There are 4 environment exposure bands - exposure band 1 has the lowest level of environmental exposure and exposure band 4 the highest level. Follow steps on this page to work out your environment exposure band.
What's your environment exposure band?
Start with Exposure Band 1 and work down the page.
Exposure band 1 criteria
If the environment categorisation volume for your chemical does not exceed 25kg, then your introduction is in environment exposure band 1. Next, work out the environment hazard characteristics of your introduction (Step 5.4)
If your introduction does not meet this criteria, go to exposure band 2 criteria.
Exposure band 2 criteria
If the environment categorisation volume for your chemical is greater than 25kg, but no more than 1,000kg, then your introduction is in environment exposure band 2. Next, work out the environment hazard characteristics of your introduction (Step 5.4)
If your introduction does not meet this criteria, go to exposure band 3 criteria.
Exposure band 3 criteria
If the environment categorisation volume for your chemical is greater than 1,000kg, but no more than 10,000kg, then your introduction is in environment exposure band 3. Next, work out the environment hazard characteristics of your introduction (Step 5.4).
If you are not in exposure bands 1-3 for the environment, go to exposure band 4 criteria.
Exposure band 4 criteria
Introductions with a designated kind of release into the environment: if you're introducing one of these you are in exposure band 4 for the environment. Next, go to Step 5.4: Work out your environment hazard characteristics.
The environment categorisation volume of your introduction is greater than 10,000kg. Go to Step 5.4: Work out your environment hazard characteristics.
Step 5.4 Work out your environment hazard characteristics
You must have permission to use information that you relied on to demonstrate the absence of hazard characteristics. If we ask you for the information that you relied on to categorise your introduction, you need to provide us with the detailed information, including full study reports, of the kind we specify in this step to demonstrate the absence of the hazard characteristics.
Hazard characteristics in environment hazard bands
A chemical has an environment hazard characteristic if the chemical can cause damage, harm or adverse effects to the environment. For example, a chemical that has the 'toxic to any aquatic life' hazard characteristic can cause toxic injury to an organism following short term aquatic exposure.
Environment hazard characteristics are split up into hazard bands. Hazard characteristics of most concern are in hazard band D, while those of lower concern are in hazard band A.
See links below to each of the hazard bands: D, C, B and A.
Our pages for environment hazard bands D, C, B and A describe hazard characteristics (eg toxic to any aquatic life and so on) in each hazard band and the information you need to have to prove your chemical does not have a particular hazard characteristic.
Information you need and hazard characteristics you need to consider
This varies depending on your introduction’s environment exposure band.
If your introduction is in a lower exposure band
Generally, in the lower exposure bands, where the level of exposure to the environment is relatively low, as a minimum you have to consider only a few hazard characteristics and you don’t need much information on them.
If your introduction is in a higher exposure band
In comparison, in higher exposure bands, where the level of exposure to the environment is higher, generally you’ll need to consider more hazard characteristics and need more information on them.
Information you need for lower indicative risk
You will need more hazard information to be able to get to lower indicative risk outcomes. Generally, within any given environment exposure band you need:
- less hazard information to get to medium to high risk
- more hazard information to get to low risk
- the most hazard information to get to very low risk
See Step 5.5 for more information about indicative environment risk outcomes
Where to start and when you can stop considering your chemical's hazard characteristics
Always start in the highest hazard band (hazard band D) and work your way down the hazard bands as far as you need to get to your outcome (that is, D, C then B then A).
You must consider each hazard characteristic in the hazard band you are in (unless there is a reason for you to stop sooner) - does your chemical have that hazard characteristic or not?
When you might not need to consider all of the hazard bands
- Because your introduction’s environment exposure band (which you worked out in step 5.3) doesn’t require it. For example, if your introduction’s environment exposure band is 2, you only need to consider the hazards in hazard band D to get to an indicative environment risk of either medium to high or low.
- Because the outcome for indicative environment risk that you are trying to get to doesn’t require it. For example, if your introduction’s environment exposure band is 3 and you want to get to an indicative environment risk of low, you only need to consider the hazard characteristics in environment hazard bands D and C.
In many cases, you’ll only need to consider hazard band D. But in other cases you might need to consider D, C, B and A because your introduction is in exposure band 3 or 4 and you are trying to get to very low indicative environment risk.
See step 5.5 for more about indicative environment risk outcomes
When you can stop working through your chemical’s environment hazard characteristics
Stop if you:
- determine that your chemical has a hazard characteristic in the hazard band (e.g. persistent, bioaccumulative and toxic - you are in hazard band D) or
- cannot demonstrate that your chemical does not have a certain hazard characteristic in that hazard band . This means that we consider your chemical to have this hazard characteristic or
- get to an indicative environment risk outcome and don’t want to go any further - see step 5.5 for more information about environment risk outcomes or
- have demonstrated that your chemical does not have any hazard characteristics in hazard bands D, C, B and A . This would only be needed for environment exposure bands 3 and 4. It means that the indicative environment risk of your introduction is very low
After you stop, you don’t need to consider the remaining hazard characteristics in the hazard band where you stopped, or any of the hazard characteristics in lower hazard bands. Take note of where and why you stopped and move on to step 5.5.
Example: Rosemary's introduction is in environment exposure band 4. She considers all of the hazard characteristics in environment hazard band D and can demonstrate that her chemical does not have any of these hazards. Rosemary then moves on to hazard band C. She works through the hazard characteristics in this hazard band in the order that they are shown in the table. When Rosemary comes to 'very toxic to any aquatic life', she finds that her chemical has this hazard characteristic. This means Rosemary can stop there. The indicative environment risk of Rosemary's introduction is medium to high. She does not need to continue further to see if her chemical has the other hazard characteristic in hazard band C (persistent and bio-accumulative). Also Rosemary doesn't need to consider if her chemical has any of the hazard characteristics in hazard bands B or A.
How to consider each hazard characteristic
Look at whether your chemical meets the hazard characteristic definition based on the information that you have.
If it does meet the hazard characteristic definition, stop there and move to step 5.5.
If it does not meet the hazard characteristic definition, you’ll need to try and prove that your chemical does not have this hazard characteristic.
Our pages on hazard bands D, C, B and A describe hazard characteristics and the ways to prove that your chemical does not have a hazard characteristic.
How to prove that your chemical does not have a hazard characteristic
You can read about your options to prove that your chemical does not have a particular hazard characteristic on each environment hazard band page. These options include:
- checking if your chemical is on the list of chemicals with high hazards for categorisation
- in silico predictions
- in vitro test results
- in vivo test results
- suitable read-across information in place of information on the chemical itself
- other information about your chemical that means that testing and in silico predictions are not necessary (that is, information waivers)
If you have access to existing information on the chemical or suitable read-across information, you should consider these first. If you need to generate new data to prove the absence of a hazard characteristic, you should choose non-animal test data when possible. You should only generate new animal test data as a last resort.
See our section on use of animal test data
If you can prove that your chemical does not have the hazard characteristic, move on to the next hazard characteristic in that hazard band, or from the next hazard band down.
If you cannot prove that your chemical does not have the hazard characteristic, stop there – your chemical is considered to have this hazard characteristic.
Take note of the hazard band that this hazard characteristic is in. If your chemical is one of these:
there may be different requirements for you to prove that your chemical does not have certain hazard characteristics.
Resources to help you with this step
We refer to the following throughout this step:
- List of chemicals with high hazards for categorisation
- In silico information - an overview of which human health (4.4) and environment characteristics have in silico options and which in silico models are appropriate.
You can also check our glossary for the definition of in silico. - Acceptable test guidelines for each environment hazard characteristic in this step
- Suitable read across information
- Decision tools for step 5.4 (self-guided tools to help you categorise your introduction)
- hazard characteristics for environment exposure band 1, hazard characteristics for environment exposure band 2, hazard characteristics for environment exposure band 3, hazard characteristics for environment exposure band 4 - use this tool if your introduction is in this exposure band
To work out the environment characteristics your chemical does and does not have, you must know your environment exposure band (Step 5.3). The information you need to consider hazard characteristics varies depending on your introduction’s exposure band.
Environment hazard bands - what are the hazard characteristics in each hazard band?
The following pages describe the hazard characteristics in each hazard band and the information you need to have to prove your chemical does not have a particular hazard characteristic. Follow instructions on each of these pages. Always start with hazard band D.
Environment hazard band D hazard characteristics
Environment hazard characteristics are split into hazard bands. Hazard characteristics of most concern are in hazard band D, while those of lower concern are in hazard band A.
Hazard band D has 5 hazard characteristics you need to consider:
- Contains arsenic, cadmium, lead or mercury
- Ozone depleting chemical
- Synthetic greenhouse gas
- Adverse effects mediated by an endocrine mode of action
- Persistent, bioaccumulative and toxic
Instructions
You must always start at hazard band D. Step 5.4 tells you when you can stop working through your chemical's environment hazard characteristics and when you need to check each of them - ie D, C, B and A.
Work your way through hazard characteristic on this page. Look at whether your chemical meets the hazard characteristic definition based on the information that you have.
If it does meet the hazard characteristic definition, stop there - your introduction's environment hazard band is D. Move on to the next step - step 5.5 Work out your environment risk for categorisation.
If it does not meet the hazard characteristic definition, you’ll need to try and prove that your chemical does not have this hazard characteristic. The information that you need to prove this for each hazard characteristic is shown below. If you do not have this information, stop there - your introduction’s environment hazard band is D. Move onto the next step – step 5.5 Work out your environment risk for categorisation.
If you do have this information (so you can prove that the chemical does not have the hazard characteristic), move onto the next hazard characteristic on this page.
After you have considered all the hazard characteristics on this page and have proven that the chemical does not have any of them, decide whether you can stop there or continue to environment hazard band C. This depends on the exposure band of your introduction.
If your introduction is in environment exposure band 1 or 2, you can choose to stop (and go to step 5.5 to work out your environment risk for categorisation), or to continue to environment hazard band C.
If your introduction is in environment exposure band 3 or 4, continue to environment hazard band C.
Hazard characteristics and required information
Contains arsenic, cadmium, lead or mercury
Contains arsenic, cadmium, lead or mercury means that the industrial chemical contains one or more of the following:
- arsenic
- cadmium
- lead or
- mercury
There are no extra information requirements to prove that the chemical does not have this hazard characteristic.
Ozone depleting chemical
Ozone depleting chemical means that any of the following apply to the industrial chemical:
- the chemical is controlled under the Ozone Protection and Synthetic Greenhouse Gas Management Act 1989, or
- the chemical is controlled under the Montreal Protocol on Substances that Deplete the Ozone Layer.
Synthetic greenhouse gas
Synthetic greenhouse gas means that any of the following apply to the industrial chemical:
- the chemical is controlled under the Ozone Protection and Synthetic Greenhouse Gas Management Act 1989, or
- the chemical is listed on the Kyoto Protocol, Synthetic Greenhouse Gases under Annex A, or
- the chemical is controlled under the Montreal Protocol on Substances that Deplete the Ozone Layer.
Adverse effects mediated by an endocrine mode of action
Adverse effects mediated by an endocrine mode of action means that any of the following apply to the industrial chemical:
- the chemical meets all of the following:
- it shows an adverse effect in an intact organism or its progeny, which is a change in the morphology, physiology, growth, development, reproduction or lifespan of an organism, system or (sub)population that results in an impairment of functional capacity, an impairment of the capacity to compensate for additional stress or an increase in the susceptibility to other influences, and
- it has an endocrine activity, which is the capacity to alter the function(s) of the endocrine system, and
- the adverse effect is a consequence of the endocrine activity
or
- the chemical is on the list of chemicals with high hazards for categorisation, based on its adverse effects mediated by an endocrine mode of action, or
- the chemical is an ester or a salt of a chemical that is listed in the table below, which are on the list of chemicals with high hazards for categorisation based on adverse effects mediated by an endocrine mode of action unless an exception, as identified in the table below, is met for that chemical,
or
- the chemical meets all of the following:
- information is available that is relevant to determining whether the chemical has the hazard characteristic, adverse effects mediated by an endocrine mode of action, and
- the information has been considered in a weight of evidence analysis based on the following guidance documents:
- the EU guidance for identifying endocrine disruptors*, and
- the guidance provided in OECD GD 150**; and
- the weight of evidence analysis concludes that the chemical has the hazard characteristic, adverse effects mediated by an endocrine mode of action.
Information required to demonstrate the absence of the hazard characteristic, adverse effects mediated by an endocrine mode of action
- If the chemical has existing information relevant to determining whether it has the hazard characteristic, adverse effects mediated by an endocrine mode of action, information is required to demonstrate that the chemical does not have this hazard characteristic:
- this must involve a documented weight of evidence analysis based on the EU guidance for identifying endocrine disruptors* and the guidance in OECD Revised Guidance Document 150, and
- the analysis must conclude that the chemical does not have the hazard characteristic, adverse effects mediated by an endocrine mode of action.
- Otherwise, the information required to demonstrate that a chemical does not have the hazard characteristic, adverse effects mediated by an endocrine mode of action, is confirmation that the chemical is not on the list of chemicals with high hazards for categorisation, based on its adverse effects mediated by an endocrine mode of action and
- Confirmation that the chemical is not an ester or salt of the specified chemicals shown in the table below, which are on the list of chemicals with high hazards for categorisation, based on adverse effects mediated by an endocrine mode of action.
Adverse effects mediated by an endocrine mode of action - exception criteria
Check the following table - an ester or salt of the chemical has the adverse effects mediated by an endocrine mode of action hazard characteristic, unless one or more of the following exception criteria apply.
| CAS number | Chemical name | An ester or salt of the chemical has the adverse effects mediated by an endocrine mode of action hazard characteristic, unless one or more of the below exception criteria apply |
|---|---|---|
| 80-05-7 | Phenol, 4,4'-(1-methylethylidene)bis- (Bisphenol A) |
|
| 80-09-1 | Phenol, 4,4'-sulfonylbis- (Bisphenol S) |
|
| 98-54-4 | Phenol, 4-(1,1-dimethylethyl)- |
|
| 80-46-6 | Phenol, 4-(1,1-dimethylpropyl)- |
|
| Various | Heptylphenols on the list of chemicals with high hazards for categorisation – includes linear and branched isomers |
|
| Various | Octylphenols on the list of chemicals with high hazards for categorisation – includes linear and branched isomers |
|
| Various | Nonylphenols on the list of chemicals with high hazards for categorisation – includes linear and branched isomers |
|
| Various | Dodecylphenols on the list of chemicals with high hazards for categorisation – includes linear and branched isomers |
|
| Various | Octyl and Nonylphenol ethoxylates on the list of chemicals with high hazards for categorisation | No exceptions |
Note – Low levels of low molecular weight species (in relation to a polymer) means less than 10% (by mass) of molecules with a molecular weight that is less than 500 g/mol and less than 25% (by mass) of molecules with a molecular weight that is less than 1,000 g/mol.
Persistent, bioaccumulative and toxic
Your introduction is in environment hazard band D if any of the following apply to the industrial chemical:
- the chemical is on the list of chemicals with high hazards for categorisation, based on it being persistent, bioaccumulative and toxic, or
- all of the following apply:
- the chemical is persistent (see our glossary definition)
- the chemical is bioaccumulative, and
- the chemical has the hazard characteristic, very toxic to any aquatic life
For the purposes of this hazard characteristic, bioaccumulative means any of the following apply to the chemical:
- it has a bioaccumulation factor (BAF) greater than or equal to 2000 for the aquatic compartment, or
- it has a bioconcentration factor (BCF) greater than or equal to 2000 for the aquatic compartment, or
- it has a measured log Kow greater than or equal to 4.2 for the aquatic compartment (unless a measured BAF or BCF is less than 2000), or
- it has a log Koa greater than 6 and log Kow greater than or equal to 2 for the terrestrial compartment, or
- it has a biomagnification factor (BMF) greater than 1.
Information required to demonstrate that a chemical does not have the hazard characteristic, persistent, bioaccumulative and toxic
Confirmation that the chemical is not on the list of chemicals with high hazards for categorisation based on it being persistent, bioaccumulative and toxic. In addition, if the environment exposure band for the introduction is 2 (and you are seeking to demonstrate that the introduction meets the criteria for very low risk and it is not the 'special cases' mentioned in step 5.5), or 3, or 4, the information required to demonstrate that a chemical does not have the hazard characteristic, persistent, bioaccumulative and toxic, is at least one of the following:
- information that demonstrates that the chemical is an inorganic chemical, or
- information to demonstrate that the chemical is a biological chemical, or
- information that demonstrates that the chemical has a molecular weight that is greater than 1,000 g/mol, or
- information that demonstrates that the chemical is a high molecular weight polymer with:
- less than 25% low molecular weight oligomeric species less than 1,000g/mol, and
- less than 10% low molecular weight oligomeric species less than 500g/mol, or
- information that demonstrates that the chemical has a solubility in water that is greater than 5g/L, measured following an acceptable test guideline for water solubility, or
- information that demonstrates that the chemical is a gas that is not expected to partition to the aquatic compartment, or
- information that demonstrates that the chemical is a substance covered by Entry 9 of Annex V of the REACH Regulation, or
- a suitable in silico prediction for partition coefficient of the chemical itself of log Kow less than 4.2 (that is not negated by a measured log Kow), or
- measured value from a study on the chemical or from suitable read-across information, conducted following an acceptable test guideline for partition coefficient, for which log Kow less than 4.2, or
- if the chemical is not a highly branched organic chemical* – a test result from a study on the chemical or from suitable read across information, conducted following an acceptable test guideline for ready biodegradability, which meets at least one of the following degradation pass levels during the period specified in the test method:
- tests based on dissolved organic carbon (DOC) - greater than or equal to 70% DOC removal, or
- tests based on carbon dioxide generation - greater than or equal to 60% theoretical carbon dioxide, or
- tests based on oxygen depletion - greater than or equal to 60% theoretical oxygen demand, or
- a test result from a study on the chemical, conducted following an acceptable test guideline for ready biodegradability, which meets at least one of the following degradation pass levels during the period specified in the test method:
- tests based on dissolved organic carbon (DOC) - greater than or equal to 70% DOC removal, or
- tests based on carbon dioxide generation - greater than or equal to 60% theoretical carbon dioxide, or
- tests based on oxygen depletion - greater than or equal to 60% theoretical oxygen demand, or
- if the chemical is not a highly branched organic chemical* – a test result from a study on the chemical or from suitable read across information, conducted following an acceptable test guideline for transformation in aquatic sediment systems, results in both:
- a degradation half-life in water of less than 2 months, and
- a degradation half-life in sediment of less than 6 months, or
- a test result from a study on the chemical, conducted following an acceptable test guideline for transformation in aquatic sediment systems, results in both:
- a degradation half-life in water of less than 2 months, and
- a degradation half-life in sediment of less than 6 months, or
- if the chemical is not a biocidal active and not a persistent, highly branched organic chemical** – information on aquatic toxicity for all 3 trophic levels (fish, invertebrates and algae), from suitable in silico predictions on the chemical or in vivo studies on the chemical or from suitable read-across information conducted following acceptable test guidelines for aquatic toxicity, with at least one of the following results for all 3 trophic levels:
- acute aquatic toxicity greater than 1 mg/L (96h LC50 (fish), or 48h EC50 (invertebrates) or 72 or 96h ErC50 (algae)), or
- chronic aquatic toxicity NOEC or EC10 greater than 0.1mg/L (for chemicals that are not readily biodegradable), or
- chronic aquatic toxicity NOEC or EC10 > 0.01 mg/L (for chemicals that are readily biodegradable), or
- test results for all 3 trophic levels (fish, invertebrates and algae) from in vivo studies on the chemical or from suitable read-across information, conducted following acceptable test guidelines for chronic aquatic toxicity with a NOEC or EC10 greater than 0.1mg/L for all 3 trophic levels, or
- a test result from an in vivo study on the chemical or from suitable read-across information, conducted following an acceptable test guideline for bioconcentration, for which the BCF less than 2,000, or
- a test result from an in vivo study on the chemical or from suitable read-across information, conducted following an acceptable test guideline for bioaccumulation, for which the BAF less than 2,000.
*If the chemical is a highly branched organic chemical, in silico predictions and read across information cannot be used to demonstrate that the chemical does not have the persistence aspect of the persistent, bioaccumulative and toxic hazard characteristic – only studies on the chemical itself, as described in the next dot point, are acceptable
**If the chemical is a biocidal active or a persistent, highly branched organic chemical, in silico predictions cannot be used to demonstrate that the chemical does not have the toxicity aspect of the persistent, bioaccumulative and toxic hazard characteristic – only in vivo chronic aquatic toxicity studies, as described in the next dot point, are acceptable.
This page accompanies step 5.4 Work out environment hazard characteristics.
Environment hazard band C hazard characteristics
Environment hazard characteristics are split into hazard bands. Hazard characteristics of most concern are in hazard band D, while those of lower concern are in hazard band A.
Hazard band C has 2 hazard characteristics you need to consider:
- very toxic to any aquatic life
- persistent and bioaccumlative - environment hazard band C
Instructions
You must always start at hazard band D. Step 5.4 tells you when you can stop working through your chemical's environment hazard characteristics and when you need to check each of them - ie D, C, B and A.. You only need to work through the hazard characteristics on this page is your introduction is in:
- Environment exposure band 1 or 2 and you are trying to get to an outcome of very low indicative environment risk or
- Environment exposure band 3 or 4
Work your way through each hazard characteristic on this page. Look at whether your chemical meets the hazard characteristic definition based on the information that you have.
If it does meet the hazard characteristic definition, stop there - your introduction's environment hazard band is C. Move on to the next step - step 5.5 Work out your environment risk for categorisation.
If it does not meet the hazard characteristic definition, you’ll need to try and prove that your chemical does not have this hazard characteristic. The information that you need to prove this for each hazard characteristic is shown below. If you do not have this information, stop there - your introduction’s environment hazard band is C. Move onto the next step – step 5.5 Work out your environment risk for categorisation.
If you do have this information (so you can prove that the chemical does not have the hazard characteristic), move onto the next hazard characteristic on this page.
After you have considered all the hazard characteristics on this page and have proven that the chemical does not have any of them, decide whether you can stop there or continue to environment hazard band B. This depends on the exposure band of your introduction.
If your introduction is in environment exposure band 1, stop here – you don’t need to consider any other hazard characteristics. Next go to step 5.5 to work out your environment risk for categorisation.
If your introduction is in environment exposure band 2, continue to environment hazard band B.
If your introduction is in environment exposure band 3, you can choose to stop here (and go to step 5.5 to work out your environment risk for categorisation, or to continue to environment hazard band B.
If your introduction is in environment exposure band 4, continue to environment hazard band B.
This page accompanies step 5.4 Work out environment hazard characteristics.
Very toxic to any aquatic life
Very toxic to any aquatic life means that any of the following apply to the industrial chemical:
- the chemical is known to cause:
- toxic injury to an organism following short term aquatic exposure as described in chapter 4.1 of the GHS, with the chemical classified as acute aquatic toxicity (category 1), or
- adverse effects to an organism during aquatic exposures determined in relation to the life-cycle of the organism, as described in chapter 4.1 of the GHS, with the chemical classified as chronic aquatic toxicity (category 1), or
- the chemical is on the list of chemicals with high hazards for categorisation based on it being very toxic to any aquatic life, or
- an in vivo acute study on the chemical:
- conducted following an acceptable test guideline for acute toxicity to fish results in a 96h LC50 less than or equal to 1 mg/L, or
- conducted following an acceptable test guideline for acute toxicity to invertebrates results in a 48h EC50 less than or equal to 1 mg/L, or
- conducted following an acceptable test guideline for acute toxicity to algae or other aquatic plants results in a 72 or 96h ErC50 less than or equal to 1 mg/L, or
- an in vivo chronic study on the chemical conducted following an acceptable test guideline for chronic toxicity to fish, chronic toxicity to invertebrates, or chronic toxicity to algae or other aquatic plants results in a:
- NOEC or EC10 less than equal to 0.1 mg/L (for chemicals that are not readily biodegradable), or
- NOEC or EC10 less than or equal to 0.01 mg/L (for chemicals that are readily biodegradable), or
- a suitable in silico prediction for acute aquatic toxicity results in a prediction of:
- for fish - 96h LC50 less than or equal to 1 mg/L, or
- for invertebrates - 48h EC50 less than or equal to 1 mg/L, or
- for algae or other aquatic plants - 72 or 96h ErC50 less than or equal to 1 mg/L
and the predictions have not been negated by in vivo studies conducted on the chemical for aquatic toxicity.
Information required to demonstrate the absence of the hazard characteristic, very toxic to any aquatic life
The information required to demonstrate that a chemical does not have the hazard characteristic, very toxic to any aquatic life, is:
- if the exposure band for the introduction is 1 - confirmation that the chemical is not on the list of chemicals with high hazards for categorisation based on it being very toxic to any aquatic life
- if the environment exposure band for the introduction is 2, 3, or 4, - at least one of the following:
- information that demonstrates that the chemical has a molecular weight greater than 1,000g/mol and has a low cationic density, or
- information that demonstrates that the chemical is a high molecular weight polymer that has a low cationic density, or
- information that demonstrates that the chemical is a substance covered by Entry 9 of Annex V of the REACH Regulation, or
- if the chemical is not a biocidal active and not a persistent, highly branched organic chemical – information on aquatic toxicity for all 3 trophic levels (fish, invertebrates and algae), from suitable in silico predictions on the chemical or in vivo studies on the chemical or from suitable read-across information conducted following acceptable test guidelines for aquatic toxicity, with one of the following results for each of the 3 trophic levels:
- acute aquatic toxicity greater than 1 mg/L (LC50 (fish), or EC50 (invertebrates) or ErC50 (algae)), or
- chronic aquatic toxicity NOEC or EC10 greater than 0.1 mg/L (for chemicals that are not readily biodegradable), or
- chronic aquatic toxicity NOEC or EC10 greater than 0.01 mg/L (for chemicals that are readily biodegradable), or
- test results for all 3 trophic levels (fish, invertebrates and algae) from in vivo studies on the chemical or from suitable read-across information, conducted following acceptable test guidelines for chronic aquatic toxicity with the following results for all 3 trophic levels:
- NOEC or EC10 greater than 0.1 mg/L (for chemicals that are not readily biodegradable), or
- NOEC or EC10 greater than 0.01 mg/L (for chemicals that are readily biodegradable).
Persistent and bioaccumulative
Persistent and bioaccumulative means that any of the following apply to the industrial chemical:
- the chemical is on the list of chemicals with high hazards for categorisation, based on it being persistent and bioaccumulative, or
- both of the following apply:
- the chemical is persistent, and
- the chemical is bioaccumulative.
For the purposes of this hazard characteristic, bioaccumulative means any of the following apply to the chemical:
- it has a bioaccumulation factor (BAF) greater than OR equal to 2000 for the aquatic compartment, or
- it has a bioconcentration factor (BCF) greater than or equal to 2000 for the aquatic compartment, or
- it has a measured log Kow greater than or equal to 4.2 for the aquatic compartment (unless a measured BCF or BAF is less than 2000), or
- it has a log Koa greater than 6 and log Kow greater than or equal to 2 for the terrestrial compartment, or
- it has a biomagnification factor (BMF) greater than 1.
Information required to demonstrate the absence of the hazard characteristic, persistent and bioaccumulative
The information required to demonstrate that a chemical does not have the hazard characteristic, persistent and bioaccumulative, is confirmation that the chemical is not on the list of chemicals with high hazards for categorisation based on it being persistent and bioaccumulative. In addition, if the environment exposure band for the introduction is 2 (and you are seeking to demonstrate that the introduction meets the criteria for very low risk and it is not the 'special cases' mentioned in step 5.5), or 3 or 4, the information required to demonstrate that a chemical does not have the hazard characteristic, persistent and bioaccumulative, is at least one of the following:
- information that demonstrates that the chemical is an inorganic chemical, or
- to demonstrate that the chemical is a biological chemical, or
- information that demonstrates that the chemical has a molecular weight that is greater than 1,000 g/mol, or
- information that demonstrates that the chemical is a high molecular weight polymer with:
- less than 25% low molecular weight oligomeric species less than 1,000g/mol, and
- less than 10% low molecular weight oligomeric species less than 500g/mol, or
- information that demonstrates that the chemical has a solubility in water that is greater than 5g/L, measured following an acceptable test guideline for water solubility, or
- information that demonstrates that the chemical is a gas that is not expected to partition to the aquatic compartment, or
- a suitable in silico prediction for partition coefficient of the chemical itself of log Kow less than 4.2 (that is not negated by a measured log Kow), or
- a measured value from a study on the chemical or from suitable read-across information, conducted following an acceptable test guideline for partition coefficient, for which log Kow less than 4.2, or
- if the chemical is not a highly branched organic chemical* – a test result from a study on the chemical or from suitable read across information, conducted following an acceptable test guideline for ready biodegradability, which meets at least one of the following degradation pass levels during the period specified in the test method:
- tests based on dissolved organic carbon (DOC) - greater than or equal to 70% DOC removal, or
- tests based on carbon dioxide generation - greater than or equal to 60% theoretical carbon dioxide, or
- tests based on oxygen depletion - greater than or equal to 60% theoretical oxygen demand, or
- a test result from a study on the chemical, conducted following an acceptable test guideline for ready biodegradability, which meets at least one of the following degradation pass levels during the period specified in the test method:
- tests based on dissolved organic carbon (DOC) - greater than or equal to 70% DOC removal, or
- tests based on carbon dioxide generation - greater than or equal to 60% theoretical carbon dioxide, or
- tests based on oxygen depletion - greater than or equal to 60% theoretical oxygen demand, or
- if the chemical is not a highly branched organic chemical* – a test result from a study on the chemical or from suitable read across information, conducted following an acceptable test guideline for transformation in aquatic sediment systems, results in both:
- a degradation half-life in water of less than 2 months, and
- a degradation half-life in sediment of less than 6 months, or
- a test result from the chemical, conducted following an acceptable test guideline for transformation in aquatic sediment systems, results in both:
- a degradation half-life in water of less than 2 months, and
- a degradation half-life in sediment of less than 6 months, or
- a test result from an in vivo study on the chemical or from suitable read-across information, conducted following an acceptable test guideline for bioconcentration, for which the BCF is less than 2,000, or
- a test result from an in vivo study on the chemical or from suitable read-across information, conducted following an acceptable test guideline for bioaccumulation, for which the BAF is less than 2,000.
*If the chemical is a biocidal active or a persistent, highly branched organic chemical, in silico predictions cannot be used to demonstrate that the chemical does not have the very toxic to any aquatic life hazard characteristic – only in vivo chronic aquatic toxicity studies, as described in the next dot point, are acceptable.
Environment hazard band B hazard characteristics
Environment hazard characteristics are split into hazard bands. Hazard characteristics of most concern are in hazard band D, while those of lower concern are in hazard band A.
Hazard band B has 1 hazard characteristic you need to consider - toxic to any aquatic life.
Instructions
You must always start at hazard band D. Step 5.4 tells you when you can stop working through your chemical's environment hazard characteristics and when you need to check each of them - ie D, C, B and A. You only need to work through the hazard characteristics on this page is your introduction is in:
- Environment exposure band 2 or 3 and you are trying to get to an outcome of very low indicative environment risk or
- Environment exposure band 4
Work your way through each hazard characteristic on this page. Look at whether your chemical meets the hazard characteristic definition based on the information that you have.
If it does meet the hazard characteristic definition, stop there - your introduction's environment hazard band is B. Move on to the next step - step 5.5 Work out your environment risk for categorisation.
If it does not meet the hazard characteristic definition, you’ll need to try and prove that your chemical does not have this hazard characteristic. The information that you need to prove this for each hazard characteristic is shown below. If you do not have this information, stop there - your introduction’s environment hazard band is B.
Move onto the next step – step 5.5 Work out your environment risk for categorisation.
If you do have this information (so you can prove that the chemical does not have the hazard characteristic), move onto the next hazard characteristic on this page.
After you have considered all the hazard characteristics on this page and have proven that the chemical does not have any of them, decide whether you can stop there or continue to environment hazard band A. This depends on the exposure band of your introduction.
If your introduction is in environment exposure band 2, stop here – you don’t need to consider any other hazard characteristics. Next go to step 5.5 to work out your environment risk for categorisation.
If your introduction is in environment exposure band 3, continue to environment hazard band A.
If your introduction is in environment exposure band 4, you can choose to stop here (and go to step 5.5 to work out your environment risk for categorisation, or to continue to environment hazard band A.
Toxic to any aquatic life
Toxic to any aquatic life means that any of the following apply to the industrial chemical:
- the chemical is known to cause:
- toxic injury to an organism following short term aquatic exposure as described in chapter 4.1 of the GHS, with the chemical classified as acute aquatic toxicity (category 2), or
- adverse effects to an organism during aquatic exposures determined in relation to the life-cycle of the organism, as described in chapter 4.1 of the GHS, with the chemical classified as chronic aquatic toxicity (category 2), or
- an in vivo acute study on the chemical:
- conducted following an acceptable test guideline for acute toxicity to fish results in a 96h LC50 greater than 1mg/L but less than or equal to 10mg/L, or
- conducted following an acceptable test guideline for acute toxicity to invertebrates results in a 48h EC50 greater than 1mg/L but less than or equal to 10mg/L, or
- conducted following an acceptable test guideline for acute toxicity to algae or other aquatic plants results in a 72 or 96h ErC50 greater than 1mg/L but less than or equal to 10mg/L, or
- an in vivo chronic study on the chemical conducted following an acceptable test guideline for chronic toxicity to fish, chronic toxicity to invertebrates, or chronic toxicity to algae or other aquatic plants results in a:
- NOEC or EC10 greater than 0.1mg/L but less than or equal to 1mg/L (for chemicals that are not readily biodegradable), or
- NOEC or EC10 greater than 0.01mg/L but less than or equal to 0.1mg/L (for chemicals that are readily biodegradable), or
- a suitable in silico prediction for acute aquatic toxicity results in a prediction of:
- for fish - 96h LC50 greater than 1mg/L but less than or equal to 10mg/L, or
- for invertebrates - 48h EC50 greater than 1mg/L but less than or equal to 10mg/L, or
- for algae or other aquatic plants - 72 or 96h ErC50 greater than 1mg/L but less than or equal to 10mg/L, or
and the predictions have not been negated by in vivo studies conducted on the chemical for aquatic toxicity.
Information required to demonstrate the absence of the hazard characteristic, toxic to any aquatic life
The information required to demonstrate that a chemical does not have the hazard characteristic, toxic to any aquatic life, is at least one of the following:
- information that demonstrates that the chemical has a molecular weight greater than 1,000 g/mol and has a low cationic density, or
- information that demonstrates that the chemical is a high molecular weight polymer that has a low cationic density, or
- information that demonstrates that the chemical is a substance covered by Entry 9 of Annex V of the REACH Regulation, or
- if the chemical is not a biocidal active and not a persistent, highly branched organic chemical – information on aquatic toxicity for all three trophic levels (fish, invertebrates and algae), from suitable in silico predictions on the chemical or in vivo studies on the chemical or from suitable read-across information conducted following acceptable test guidelines for aquatic toxicity, with the following results for all three trophic levels:
- acute aquatic toxicity greater than 10 mg/L (LC50 (fish), or EC50 (invertebrates) or ErC50 (algae)), or
- chronic aquatic toxicity NOEC or EC10 greater than 1mg/L (for chemicals that are not readily biodegradable), or
- chronic aquatic toxicity NOEC or EC10 greater than 0.1mg/L (for chemicals that are readily biodegradable), or
- test results for all three trophic levels (fish, invertebrates and algae) from in vivo studies on the chemical or from suitable read-across information, conducted following acceptable test guidelines for chronic aquatic toxicity with the following results for all three trophic levels:
- NOEC or EC10 greater than 1mg/L (for chemicals that are not readily biodegradable), or
- NOEC or EC10 greater than 0.1mg/L (for chemicals that are readily biodegradable).
This page accompanies step 5.4 Work out environment hazard characteristics.
Environment hazard band A hazard characteristics
This page accompanies step 5.4 Work out environment hazard characteristics.
Environment hazard characteristics are split into hazard bands. Hazard characteristics of most concern are in hazard band D, while those of lower concern are in hazard band A.
Hazard band A has 6 hazard characteristics you need to consider:
- Contains aluminium, chromium, copper, nickel, selenium, silver or zinc
- Polymer that does not have a low cationic density
- Polymer that is not stable
- Bioaccumulation potential
- Industrial chemical (other than a polymer) that does not meet the criteria for ready biodegradability
- Harmful to any aquatic life
Instructions
You must always start at hazard band D. Step 5.4 tells you when you can stop working through your chemical's environment hazard characteristics and when you need to check each of them - that is, D, C, B and A. You only need to work through the hazard characteristics on this page is your introduction is in:
- Environment exposure band 3 or 4 and you are trying to get to an outcome of very low indicative environment risk
Work your way through each hazard characteristic on this page. Look at whether your chemical meets the hazard characteristic definition based on the information that you have.
If it does meet the hazard characteristic definition, stop there - your introduction's environment hazard band is A. Move on to the next step - step 5.5 Work out your environment risk for categorisation.
If it does not meet the hazard characteristic definition, you’ll need to try and prove that your chemical does not have this hazard characteristic. The information that you need to prove this for each hazard characteristic is shown below. If you do not have this information, stop there - your introduction’s environment hazard band is A. Move onto the next step – step 5.5 Work out your environment risk for categorisation.
If you do have this information (so you can prove that the chemical does not have the hazard characteristic), move onto the next hazard characteristic on this page.
After you have considered all the hazard characteristics on this page and have proven that the chemical does not have any of them, go to step 5.5 to work out your environment risk for categorisation.
Links to resources to help you with the following:
- Read-across information
- Acceptable test guidelines for categorisation
- In silico predictions for categorisation
Hazard characteristics and required information
Contains aluminium, chromium, copper, nickel, selenium, silver or zinc
Contains aluminium, chromium, copper, nickel, selenium, silver or zinc means that the industrial chemical contains one or more of the following:
- aluminium
- chromium
- copper
- nickel
- selenium
- silver
- zinc
There are no extra information requirements to prove that the chemical does not have this hazard characteristic.
Polymer that does not have a low cationic density
Polymer that does not have a low cationic density means that the industrial chemical is a polymer that does not meet the definition of low cationic density.
There are no extra information requirements to prove that the chemical does not have this hazard characteristic.
Polymer that is not stable
Polymer that is not stable means that all of the following apply to the industrial chemical:
- the chemical is a polymer, and
- the polymer substantially degrades, decomposes or depolymerises during use; that is, the polymer is considerably, meaningfully or to a significantly large extent, changed into simpler, smaller molecular weight chemicals as the result of processes including, but not limited to:
- oxidation
- hydrolysis
- heat
- sunlight
- attack by solvents
Information required to demonstrate the absence of the hazard characteristic, polymer that is not stable
The information required to demonstrate that a chemical does not have the hazard characteristic, polymer that is not stable, is at least one of the following:
- information that demonstrates that the polymer is protected from degradation by being encapsulated during use, or
- information that demonstrates that all of the following applies to the polymer:
- it is not designed to be pyrolysed or burnt, and
- it is not designed or reasonably anticipated to substantially photodegrade, and
- it is not designed or reasonably anticipated to substantially biodegrade, and
- it is not explosive, and
- it is hydrolytically stable (T½ greater than or equal to 12 hours), and
- it is not a biological polymer, and
- it is not a polysaccharide, and
- if it is a polymer that contains polyethylene glycol (PEG) functionalities and has a solubility in water of greater than 200 mg/L - measured data demonstrates that the polymer does not substantially biodegrade, and
- if it is a polymer that contains polypropylene glycol (PPG) functionalities and has a solubility in water of greater than 200 mg/L - measured data demonstrates that the polymer does not substantially biodegrade.
Industrial chemical (other than a polymer) that does not meet the criteria for ready biodegradability
Industrial chemical (other than a polymer) that does not meet the criteria for ready biodegradability, means that a study on the chemical, conducted following an acceptable test guideline for ready biodegradability, results in at least one of the following, as relevant to the test method used, and within the period specified in the test method:
- less than or equal to 70% dissolved organic carbon (DOC) removal, or
- less than or equal to 60% theoretical carbon dioxide, or
- less than or equal to 60% theoretical oxygen demand.
Information required to demonstrate the absence of the hazard characteristic, industrial chemical (other than a polymer) that does not meet the criteria for ready biodegradability
The information required to demonstrate that a chemical does not have the hazard characteristic, industrial chemical (other than a polymer) that does not meet the criteria for ready biodegradability, is at least one of the following:
- information that demonstrates that the chemical is highly volatile and it is expected to predominately partition to the air compartment, or
- information that demonstrates that it is an inorganic chemical, or
- information that demonstrates that it is a biological chemical, or
- if the chemical is not a highly branched organic chemical* – a test result from a study on the chemical or suitable read across information, conducted following an acceptable test guideline for ready biodegradability, which meets at least one of the following degradation pass levels during the period specified in the test method:
- tests based on dissolved organic carbon (DOC) - greater than or equal to 70% DOC removal, or
- tests based on carbon dioxide generation - greater than or equal to 60% theoretical carbon dioxide
- tests based on oxygen depletion - greater than or equal to 60% theoretical oxygen demand, or
- a test result from a study on the chemical, conducted following an acceptable test guideline for ready biodegradability, which meets at least one of the following degradation pass levels during the period specified in the test method:
- tests based on dissolved organic carbon (DOC) - greater than or equal to 70% DOC removal, or
- tests based on carbon dioxide generation - greater than or equal to 60% theoretical carbon dioxide, or
- tests based on oxygen depletion - greater than or equal to 60% theoretical oxygen demand.
*If the chemical is a highly branched organic chemical, in silico predictions and read across information cannot be used to demonstrate that the chemical does not have the hazard characteristic, industrial chemical (other than a polymer) that does not meet the criteria for ready biodegradability – only studies on the chemical itself, as described in the next dot point, are acceptable.
Harmful to any aquatic life
Harmful to any aquatic life means that any of the following apply to the industrial chemical:
- the chemical is known to cause:
- toxic injury to an organism following short term aquatic exposure , as described in chapter 4.1 of the GHS, with the chemical classified as acute aquatic toxicity (category 3), or
- adverse effects to an organism during aquatic exposures determined in relation to the life-cycle of the organism, as described in chapter 4.1 of the GHS, with the chemical classified as chronic aquatic toxicity (category 3 or 4), or
- an in vivo acute study on the chemical:
- conducted following an acceptable test guideline for acute toxicity to fish results in a 96h LC50 greater than 10mg/L but less than or equal to 100mg/L, or
- conducted following an acceptable test guideline for acute toxicity to invertebrates results in a 48h EC50 greater than 10mg/L but less than or equal to 100mg/L, or
- conducted following an acceptable test guideline for acute toxicity to algae or other aquatic plants results in a 72 or 96h ErC50 greater than 10mg/L but less than or equal to 100mg/L, or
- an in vivo chronic study on the chemical conducted following an acceptable test guideline for chronic toxicity to fish, chronic toxicity to invertebrates, or chronic toxicity to algae or other aquatic plants results in a:
- NOEC or EC10 greater than 0.1mg/L but less than or equal to 1mg/L (for chemicals that are readily biodegradable), or
- a suitable in silico prediction for acute aquatic toxicity results in a prediction of:
- for fish - 96h LC50 greater than 10mg/L but less than or equal to 100mg/L, or
- for invertebrates - 48h EC50 greater than 10mg/L but less than or equal to 100mg/L, or
- for algae or other aquatic plants - 72 or 96h ErC50 greater than 10mg/L but less than or equal to100mg/L.
and the predictions have not been negated by in vivo studies conducted on the chemical for aquatic toxicity.
Information required to demonstrate that a chemical does not have the hazard characteristic, harmful to any aquatic life
At least one of the following:
- information that demonstrates that the chemical has a molecular weight greater than 1,000g/mol and has a low cationic density, or
- information that demonstrates that the chemical is a high molecular weight polymer that has a low cationic density, or
- information that demonstrates that the chemical is a substance covered by Entry 9 of Annex V of the REACH Regulation, or
- if the chemical is not a biocidal active and not a persistent, highly branched organic chemical – information on aquatic toxicity for all three trophic levels (fish, invertebrates and algae), from suitable in silico predictions on the chemical or in vivo studies on the chemical or from suitable read-across information conducted following acceptable test guidelines for aquatic toxicity, with the following results for all three trophic levels:
- acute aquatic toxicity greater than 100 mg/L (LC50 (fish), or EC50 (invertebrates) or ErC50 (algae)), or
- chronic aquatic toxicity NOEC or EC10 greater than 1mg/L (for chemicals that are readily biodegradable), or
- test results for all three trophic levels (fish, invertebrates and algae) from in vivo studies on the chemical or from suitable read-across information, conducted following acceptable test guidelines for chronic aquatic toxicity with the following results for all three trophic levels:
- NOEC or EC10 greater than 1mg/L (for chemicals that are readily biodegradable).
Bioaccumulation potential
Bioaccumulation potential means that at least one of the following applies to the industrial chemical:
- it has a bioconcentration factor (BCF) greater than or equal to 500, or
- it has a bioaccumulation factor (BAF) greater than or equal to 500, or
- it has a partition coefficient (log Kow) greater than or equal to 4.0 (unless a measured BAF or BCF is <500).
Information required to demonstrate the absence of the hazard characteristic, bioaccumulation potential
The information required to demonstrate that a chemical does not have the hazard characteristic, bioaccumulation potential, is at least one of the following:
- information that demonstrates that the chemical is an inorganic chemical, or
- information that demonstrates that the chemical has a high molecular weight, or
- information that demonstrates that the chemical is a high molecular weight polymer with:
- less than 25% low molecular weight oligomeric species less than 1,000g/mol
- less than 10% low molecular weight oligomeric species less than 500g/mol, or
- information that demonstrates that the chemical has a solubility in water that is greater than 5g/L, measured following an acceptable test guideline for water solubility, or
- information that demonstrates that the chemical is a gas that is not expected to partition to the aquatic compartment, or
- a measured value from a study on the chemical or from suitable read-across information, conducted following an acceptable test guideline for partition coefficient, for which log Kow less than 4.0, or
- a suitable in silico prediction for partition coefficient of the chemical using KOWWIN on the chemical for log Kow less than 4.0 (that is not negated by a measured log Kow), or
- a test result from an in vivo study on the chemical or from suitable read-across information, conducted following an acceptable test guideline for bioconcentration, for which the BCF less than 500, or
- a test result from an in vivo study on the chemical or from suitable read-across information, conducted following an acceptable test guideline for bioaccumulation, for which the BAF less than 500.
Step 5.5 Your environment risk for categorisation
Get help with this step — explore our categorisation decision tools
We explain the table in detail for each environment exposure band that your introduction could be in. This includes what your indicative environment risk outcome will be, depending on which hazard characteristics your chemical does or does not have. Your outcome will be that your introduction has an indicative environment risk of:
- Medium to high
- Low OR
- Very low
Refer back to step 5.4 for information about how to consider the hazard characteristics and where to start and stop when considering hazard characteristics.
Environment risk table
| Work out your indicative environment risk | Environment exposure band | ||||
| 1 | 2 | 3 | 4 | ||
| Environment hazard band | D | Medium to high risk | Medium to high risk | Medium to high risk | Medium to high risk |
| C | Low risk | Low risk | Medium to high risk | Medium to high risk | |
| B | Very low risk | Low risk | Low risk | Medium to high risk | |
| A | Very low risk | Very low risk | Low risk | Low risk | |
| Not A, B, C or D | Very low risk | Very low risk | Very low risk | Very low risk | |
If your introduction is environment exposure band 1
If your introduction is in environment exposure band 1, at a minimum, you will need to consider if your chemical has any of the hazard characteristics in environment hazard band D.
The indicative environment risk of your introduction will be:
- Medium to high if your chemical has 1 or more of the hazard characteristics in environment hazard bands D OR
- Low if your chemical does not have any of the hazard characteristics in environment hazard band D
You can choose to stop if you get to low indicative environment risk.
If you want to see if your introduction could have a very low indicative environment risk, you will also need to consider if it has any of the hazard characteristics in environment hazard band C.
The indicative environment risk of your introduction will be:
- Low if your chemical has 1 or more of the hazard characteristics in environment hazard band C OR
- Very low if your chemical does not have any of the hazard characteristics in environment hazard band C
If your introduction is environment exposure band 2
If your introduction is in environment exposure band 2, you will need to consider if your chemical has any of the hazard characteristics in environment hazard band D.
The indicative environment risk of your introduction will be:
- Medium to high if your chemical has 1 or more of the hazard characteristics in environment hazard band D OR
- Low if your chemical does not have any of the hazard characteristics in environment hazard band D
You can choose to stop if you get to low indicative environment risk.
If you want to see if your introduction could have a very low indicative environment risk, you will also need to consider if it has any of the hazard characteristics in environment hazard bands C and B.
The indicative environment risk of your introduction will be:
- Low if your chemical has 1 or more of the hazard characteristics in environment hazard bands C or B OR
- Very low if your chemical does not have any of the hazard characteristics in environment hazard bands C or B
If your introduction is environment exposure band 3
If your introduction is in environment exposure band 3, you will first need to consider if your chemical has any of the hazard characteristics in environment hazard band D. If it does not, then continue on to consider the hazard characteristics in environment hazard band C.
The indicative environment risk of your introduction will be:
- Medium to high if your chemical has 1 or more of the hazard characteristics in environment hazard bands D or C OR
- Low if your chemical does not have any of the hazard characteristics in environment hazard bands D or C
You can choose to stop if you get to low indicative environment risk.
If you want to see if your introduction could have a very low indicative environment risk, you will also need to consider if it has any of the hazard characteristics in environment hazard bands B and A.
The indicative environment risk of your introduction will be:
- Low if your chemical has 1 or more of the hazard characteristics in environment hazard bands B or A OR
- Very low if your chemical does not have any of the hazard characteristics in environment hazard bands B or A
If your introduction is environment exposure band 4
If your introduction is in environment exposure band 4, you will first need to consider if your chemical has any of the hazard characteristics in environment hazard band D. If it does not, then continue on to consider the hazard characteristics in environment hazard band C. If it does not, then continue on to consider the hazard characteristics in environment hazard band B.
The indicative environment risk of your introduction will be:
- Medium to high if your chemical has 1 or more of the hazard characteristics in environment hazard bands D, C or B OR
- Low if your chemical does not have any of the hazard characteristics in environment hazard bands D, C or B
You can choose to stop if you get to low indicative environment risk.
If you want to see if your introduction could have a very low indicative environment risk, you will also need to consider if it has any of the hazard characteristics in environment hazard band A.
The indicative environment risk of your introduction will be:
- Low if your chemical has 1 or more of the hazard characteristics in environment hazard band A OR
- Very low if your chemical does not have any of the hazard characteristics in environment hazard band A
'Special cases' - introductions that CANNOT have a very low indicative environment risk
Your introduction CANNOT have a very low indicative environment risk if it is a:
- organotin chemical OR
- polyhalogenated organic chemical OR
- a chemical that has an end use as a biocidal active OR
- a chemical that is introduced as a solid or a dispersion that is not soluble, that meets the nanoscale particle size criteria, and the introduction of the nanoscale portion of the chemical (the part that has a particle size range of 1nm to 100nm) is incidental to the introduction of the non-nanoscale portion OR
- chemical that is introduced as a solid or a dispersion where there is no information available on its water solubility or its particle size, and the introduction of any nanoscale portion of the chemical (the part that has a particle size range of 1nm to 100nm) is incidental to the introduction of the non-nanoscale portion
If your introduction is 1 of these, and you got a very low risk outcome in this step, you need to CHANGE that outcome to LOW RISK.
This means if your consideration of step 5.5 got you to an outcome of very low risk, your final outcome needs to be changed to low risk.
Definitions of these 'special cases'
Organotin chemicals are chemicals that contain at least 1 tin atom that is covalently bound to at least one carbon atom.
Polyhalogenated organic chemicals are carbon-based chemicals that contain more than 1 covalently bonded halogen atom, such as bromine, chlorine, fluorine or iodine.
Biocidal active is a chemical that is intended to act by chemical means on or against a harmful organism by destroying, deterring, rendering harmless, preventing the action of, or otherwise exerting a controlling effect on, the harmful organism.
Nanoscale particle size criteria means that the chemical consists of particles in an unbound state or as an aggregate or agglomerate. At least 50% (by number size distribution) of the particles must have at least 1 external dimension in the particle size range of 1nm to 100nm (i.e. the nanoscale).
Not soluble means the solubility of the chemical in water is less than 33.3 g/L measured following OECD test guidelines 105 or 120 for water solubility; or the dissolution rate of the chemical is not more than 70%.
The table on this page shows how you can work out your indicative environment risk by using your environment exposure band (step 5.3) and the environment hazard characteristics (step 5.4) that your chemical does or does not have.
Step 6: Complete your categorisation
What's my introduction's category?
Use our categorisation matrix and your outcome from steps 4.5 and 5.5 to work out if your introduction is in the exempted or reported or assessed category.
- First, find the column that corresponds with your introduction's indicative human health risk, which is either 'very low', 'low' or 'medium to high'.
- Next, move down the rows until you find your introduction's indicative environment risk, which is either 'very low', 'low' or 'medium to high'.
- Find where these intersect you have your introduction's category
It's time to work out your introduction’s category using the outcome of your introduction's indicative human health risk and environment risk.
Exempted
Your introduction is in the exempted category if the indicative risk to both human health and the environment is 'very low'.
Human health risk is very low + environment risk very low = exempted introduction
Reported
Your introduction is in the reported category if the highest indicative risk of your introduction to human health or the environment is 'low'. This means either low risk to both human health and the environment, or low risk for one and very low risk for the other.
Human health risk very low + environment risk low = reported introduction
Human health risk low + environment risk very low = reported introduction
Human health risk low + environment risk low = reported introduction
Assessed
Your introduction is in the assessed category if the highest indicative risk of your introduction to human health or the environment is 'medium to high'. This means either medium to high risk to both human health and environment, or, medium to high risk for one and low or very low risk for the other.
Human health risk very low + indicative environment risk medium to high = assessed introduction (you must apply for an assessment certificate type 'environment focus')
Human health risk low + environment risk medium to high = assessed introduction (you must apply for an assessment certificate type 'environment focus')
Human health risk medium to high + environment risk very low = assessed introduction (you must apply for an assessment certificate type 'health focus')
Human health risk medium to high + environment risk low = assessed introduction (you must apply for an assessment certificate type 'health focus')
Human health risk medium to high + indicative environment risk medium to high = assessed introduction (you must apply for an assessment certificate type 'health and environment focus')
Example: David has worked through step 4.5 and found that his introduction's indicative human health risk is low. He then worked through step 5.5 and found that the indicative risk to the environment is medium to high. The highest indicative risk for David's introduction is medium to high, therefore his introduction is categorised as assessed.
Final step
Now you know the introduction category, read 'Your obligations after categorisation' to see what you must do next, including records you must keep.
Your obligations after categorisation
If your introduction is in the listed category
Learn more about your obligations after categorisation for listed introductions.
If your introduction is in the exempted category
You can introduce your chemical without telling us first, if you are already registered with us. You will need to keep records about the introduction and submit an annual declaration at the end of the registration year (from August). You must also submit a once-off exempted introduction declaration if you are introducing any of the following for the first time:
- polymers of low concern
- low-concern biopolymers
- introductions that you have categorised as very low risk for human health and the environment at steps 4-6 of the categorisation process.
If your introduction is in the reported category
You can introduce your chemical into Australia as a reported introduction, as long as you are registered with us and you submit a once-off pre-introduction report before you introduce the chemical.
You must also keep records about the introduction, which varies based on the type of reported introduction. You must also submit an annual declaration at the end of the registration year (from August).
If your introduction is in the assessed category
You must be registered with us and apply for an assessment certificate. We will assess your chemical introduction. You can start introducing after we issue an assessment certificate.
You must also keep records about the introduction and submit an annual declaration at the end of the registration year (from August).
For chemicals that are already on the Inventory, you must apply to vary the terms of the Inventory listing (if applicable).
Your obligations after categorisation for listed introductions
Once you’ve worked out that your introduction is in the listed category, go through A – E on this page to learn about your obligations.
A. Register your business
You must register your business with us and keep records to prove that you are registered at the correct level.
Once you have completed your registration, you can start manufacturing or importing your chemical.
B. Keep records about your listed introduction
You must keep records about the chemicals that you import or manufacture (introduce) under the listed category.
C. Annual declaration
You must submit an annual declaration at the end of each registration year (from August). Select ‘Listed introduction’ in your declaration form.
Other ongoing obligations that apply
Your chemical introduction will remain a listed introduction as long as:
- The chemical is on the Inventory
- The importation or manufacture of the chemical is within the terms of Inventory listing.
Important – if the circumstances of your introduction change, make sure you check that the introduction of your chemical is within the terms of Inventory listing.
D. If the chemical’s Inventory listing has conditions, a defined scope of assessment or specific information requirement
If the circumstances of your introduction change, work through step 0 again to make sure your introduction is still a listed introduction. If the Inventory listing has a specific information requirement, use our guide to work out if you must submit information in AICIS Business Services.
E. Report hazard information on chemicals previously assessed by AICIS
Introducers must report hazard information that they became aware of since the most recent publication of an AICIS assessment or evaluation statement on the chemical.
If a person who has introduced a chemical within the previous 12 months becomes aware of information about a new or increased hazard to human health or the environment from the introduction or use of a chemical, then they must report this to AICIS within 20 days of becoming aware of the information.
You must provide information about:
- any hazard to human health or the environment from the introduction or use of the industrial chemical that is not identified in the most recent AICIS assessment statement or evaluation statement on that chemical
- any hazard to human health or the environment that is identified in the most recent assessment statement or evaluation statement for the industrial chemical and that indicates an increase in the severity of the hazard.
Appendix – In silico predictions for categorisation
These tables are an overview on which human health and environment hazard characteristics have in silico options, and which in silico options are appropriate.
In silico models for human health hazard characteristics
| In silico model | Acute toxicity | Skin irritation / Skin corrosion | Eye damage / Eye irritation | Skin sensitisation | Respiratory sensitisation | Genetic toxicity |
|---|---|---|---|---|---|---|
| OECD QSAR Toolbox | Yes | Yes | Yes | Yes | Yes | Yes |
| VEGA QSAR | - | - | - | Yes | - | Yes |
| Danish EPA QSAR Database | Yes | Yes | - | Yes | Yes | Yes |
| T.E.S.T. | Yes | - | - | - | - | Yes |
| ToxTree | - | Yes | Yes | Yes | Yes (as protein binding alerts) | Yes |
| Derek Nexus | - | Yes | Yes | Yes | Yes | Yes |
| Sarah Nexus | - | - | - | - | - | Yes |
| OASIS-TIMES | Yes | Yes | Yes | Yes | - | Yes |
| Chemtunes | Yes | - | - | Yes | - | Yes |
| Case ULTRA | Yes | Yes | Yes | Yes | - | Yes |
| ADMET Predictor | - | - | - | Yes | Yes | Yes |
| Biovia Discovery Studio (TOPKAT) | Yes | Yes | Yes | Yes | - | Yes |
| ACD Percepta | Yes | Yes | Yes | - | - | Yes |
| Hazard Expert | - | - | - | - | - | Yes |
| Cheminformatics Tool Kit | Yes | Yes | Yes | Yes | - | Yes |
| Toxread | - | - | - | - | - | Yes |
| PaDEL-DDPredictor | - | Yes | Yes | - | - | - |
| Tox21 | - | - | - | Yes | - | - |
| iSafeRat® Desktop | - | - | Yes | - | - | - |
In silico models for environment hazard characteristics
| In silico model | Acute aquatic toxicity | Chronic aquatic toxicity | Persistence (as a function of half-life) | Bioaccumulation (as a function of Log Kow) |
|---|---|---|---|---|
| ECOSAR | Yes | - | - | - |
| EPI Suite | - | - | Yes | - |
| KOWWIN | - | - | - | Yes |
| OASIS-CATALOGIC | Yes | - | Yes | Yes |
| iSafeRat® Desktop | Yes | - | - | Yes |
Use information on this page to help you complete steps 4.4 and 5.4 to work out the human health hazard characteristics and environment hazard characteristics of your introduction.
Appendix – acceptable test guidelines for categorisation
The acceptable test guidelines for each hazard characteristic and property are set out in the tables below. They include:
- current Organisation for Economic Cooperation and Development (OECD) test guidelines (and their adopted versions if the version shown in the table below is only a draft version)
- deleted and superseded OECD test guidelines if the study was done before the guideline was deleted or superseded
- US EPA OPPT (Office of Prevention, Pesticides and Toxic Substances) test guidelines
- US EPA OCSPP (Office of Chemical Safety and Pollution Prevention) Harmonised Test Guidelines
- test methods for EU REACH, set out in Council Regulation (EC) No 440/2008 (Test Methods Regulation).
Acceptable test guidelines for human health hazard characteristics
| Hazard tested | OECD test guidelines | Equivalent test guidelines |
|---|---|---|
| Acute dermal toxicity – in vivo | 402 or draft 434 | EU Annex V test method B.3 OCSPP 870.1200, OPPT 798.110, OPP 81-2 |
| Acute inhalation toxicity – in vivo | 403 or 436 or draft 433 | EU Annex V test methods B.2 or B.52 OCSPP 870.1300, OPPT 798.1150, OPP 81-3 |
| Acute oral toxicity – in vivo | 420, 423, 425 or deleted 401 | EU Annex V test methods B.1, B.1 bis, B.1 tris OCSPP 870.1100, OPPT 798.1175, OPP 81-1 |
| Acute oral toxicity – in vitro | - | OECD Series on Testing and Assessment, No. 129, Guidance Document on Using Cytotoxicity Tests to Estimate Starting Doses for Acute Oral Systemic Toxicity Tests (2010) |
| Carcinogenicity – in vivo | 451 | EU Annex V test method B.32 OCSPP 870.4200, OPPT 798.3300, OPP 83-2 |
| 453 | EU Annex V test method B.33 OCSPP 870.4300, OPPT 798.3320, OPP 83-5 | |
| Chromosomal abnormalities – in vivo | 474 | EU Annex V test method B.12 OCSPP 870.5395, OPPT 798.5395, OPP 84-2 |
| 475 | EU Annex V test method B.11 OCSPP 870.5385, OPPT 798.5385, OPP 84-2 | |
| Chromosomal abnormalities – in vitro | 474 | EU Annex V test method B.12 OCSPP 870.5395, OPPT 798.5395, OPP 84-2 |
| 475 | EU Annex V test method B.11 OCSPP 870.5385, OPPT 798.5385, OPP 84-2 | |
| Chromosomal abnormalities – in vitro | 473 | EU Annex V test method B.10 OCSPP 870.5375, OPPT 798.5375, OPP 84-2 |
| 487 | EU Annex V test method B.49 | |
| 490 | - | |
| Chronic toxicity – in vivo | 452 | EU Annex V test method B.30 OCSPP 870.4100, OPPT 798.3260, OPP 83-1 |
| 453 | EU Annex V test method B.33 OCSPP 870.4300, OPPT 798.3320, OPP 83-5 | |
| Developmental toxicity – in vivo | 414 | EU Annex V test method B.31 OCSPP 870.3700, OPPT 798.4900 or OPP 83-3 |
| 426 | OCSPP 870.6300, OPP 83-6 | |
| 4221 | OCSPP 870.36501 | |
| Eye damage – in vitro | 437 | EU Annex V test method B.47 EURL ECVAM DB-ALM protocols No. 98 and 124 |
| 438 | EU Annex V test method B.48 EURL ECVAM DB-ALM protocol No. 80 | |
| 460 | EURL ECVAM DB-ALM protocol No. 71 | |
| 491 | - | |
| 494 | - | |
| Eye irritation – in vitro | 492 | - |
| Eye irritation – in vivo | 405 | EU Annex V test methods B.5 OCSPP 870.2400, OPPT 798.4500 or OPP 81-4 |
| Gene mutation – in vivo | 486 | EU Annex V test method B.39 |
| 488 | EU Annex V test method B.58 | |
| 489 | - | |
| Gene mutation – in vitro | 471 | EU Annex V test methods B.13 and B.14 OCSPP 870.5100, OPPT 798.5100, OPPT 798.5265, OPP 84-2 |
| 476 | EU Annex V test method B.17 OCSPP 870.5300, OPPT 798.5300, OPP 84-2 | |
| Heritable germ cell mutagenicity – in vivo | 478 | EU Annex V test method B.22 OPPT 798.5450, 870.5450 |
| 485 | EU Annex V test method B.25 OPPT 798.5460, 870.5460 | |
| Reproductive toxicity – in vivo | 421 | OCSPP 870.3550 |
| 4222 | OCSPP 870.36502 | |
| 443 | EU Annex V test method B.56 | |
| 415 | EU Annex V test method B.34 | |
| 416 | EU Annex V test method B.35 OCSPP 870.3800, OPPT 798.4700 or OPP 83-4 | |
| Skin corrosion – in vitro | 430 | EU Annex V test method B.43 EURL ECVAM DB-ALM protocol No.115 |
| 431 | EU Annex V test method B.40 EURL ECVAM DB-ALM protocols No.118 and 119 | |
| 435 | EURL ECVAM DB-ALM protocol No.116 | |
| Skin irritation – in vitro | 439 | EU Annex V test method B.46 EURL ECVAM DB-ALM protocols No.131, 135 and 138 |
| Skin irritation – in vivo | 404 | EU Annex V test method B.4. OCSPP 870.2500, OPPT 798.4470, OPP 81-5 |
| Skin sensitisation – defined approach | 497 | - |
| Skin sensitisation – in chemico (1st key event in skin sensitisation) | 442C | EU Annex V test methods B.59 EURL ECVAM DB-ALM protocol No.154 |
| Skin sensitisation – in vitro (2nd key event in skin sensitisation) | 442D | EU Annex V test method B.60 EURL ECVAM DB-ALM protocol No.155 |
| Skin sensitisation – in vitro (3rd key event in skin sensitisation) | 442E | EURL ECVAM DB-ALM protocol No.158 |
| Skin sensitisation – in vivo | 406 | EU Annex V test method B.6 OCSPP 870.2600, OPPT 798.4100 or OPP 81-6 |
| 429 | EU Annex V test method B.42 | |
| 442A | EU Annex V test method B.50 | |
| 442B | EU Annex V test method B.51 | |
| Subacute dermal toxicity – in vivo | 410 | EU Annex V test method B.9 OCSPP 870.3200 or OPP 82-2 |
| Subacute inhalation toxicity – in vivo | 412 | EU Annex V test method B.8 |
| Subacute oral toxicity – in vivo | 407 | EU Annex V test method B.7 OCSPP 870.3050 |
| Subchronic dermal toxicity – in vivo | 411 | EU Annex V test method B.28 OCSPP 870.3250, OPPT 798.2250, OPP 82-3 |
| Subchronic inhalation toxicity – in vivo | 413 | EU Annex V test method B.29 OCSPP 870.3465, OPPT 798.2450, OPP 82-4 |
| Subchronic oral toxicity – in vivo | 408 | EU Annex V test method B.26 OCSPP 870.3100, OPPT 798.2650, OPP 82-1 |
| 409 | EU Annex V test method B.27 OCSPP 870.3150, OPP 82-1 |
1 – Only for the purposes of the definition of 'developmental toxicity'.
2 – Only for the purposes of the definition of 'reproductive toxicity'.
Acceptable test guidelines for environment hazard characteristics and properties
| Hazard or property tested | OECD test guidelines | Equivalent test guidelines |
|---|---|---|
| Acute aquatic toxicity – in vivo (fish) | 203 | ISO 10229 EU Annex V test method C.1 OCSPP 850.1075, OPP 72-1, OPP 72-3 |
| Acute aquatic toxicity – in vivo (invertebrates) | 202 | ISO 6341 EU Annex V test method C. 2 OCSPP 850.1010 or OPP 72-2 |
| Acute aquatic toxicity – in vivo (algae or other aquatic plants) | 201 | EU Annex V test methods C.3 OCSPP 850.4550, OPPT 797.1050, OPP 122-2, OPP 123-2 |
| Bioaccumulation | 315 | OCSPP 850.1710 |
| 317 | - | |
| Bioconcentration | 305 | EU Annex V test methods C.13 OCSPP 850.1730 or OPP 72-6 |
| Chronic aquatic toxicity – in vivo (fish) | 210 | OCSPP 850.1400 EU Annex V test method C.15 |
| Chronic aquatic toxicity – in vivo (invertebrates) | 211 | OCSPP 850.1300 EU Annex V test method C.20 |
| Chronic aquatic toxicity – in vivo (algae or other aquatic plants) | 201 | OCSPP 850.4550 OCSPP 850.4500 EU Annex V test method C.3 |
| Partition coefficient | 107 | EU Annex V test methods A.8 OCSPP 830.7550, OPPT 796.1550, OPP 63-11 |
| 117 | EU Annex V test methods A.8 OCSPP 830.7570, OPPT 796.1570, OPP 63-11 | |
| 123 | EU Annex V test methods A.8 | |
| Ready biodegradability | 301 | U Annex V test methods C.4 (A-F) OCSPP 835.3110, OPPT 796.3180, 796.3200, 796.3220, 796.3240, 796.3260 |
| 310 | OPPTS 835.3140 | |
| Transformation in aquatic sediment systems | 308 | OPP 162-3, 162-4- |
Acceptable test guidelines for water solubility
| Property tested | OECD test guidelines | Equivalent test guidelines |
|---|---|---|
| Water solubility – chemicals or polymers | 105 | OPPTS 830.7840 OPPTS 830.7860 |
| Water solubility – polymers | 120 | - |
Information on this page will help you complete steps 4.4 and 5.4 in working out what the human health characteristics and environment hazard characteristics of your introduction are.
Appendix: Calculate your environment categorisation volume (ECV)
You need to work out your environment categorisation volume (ECV) when you are working out which environment exposure band applies to your introduction at step 5.3.
If your introduction has a single end use
Use either equation 1 or 2.
Equation 1: simplest method (single end use; no release reduction factor (RRF))
ECV = IV
IV = Introduction volume. This means the total importation and manufacture quantity of your chemical in kilograms (kg) in an AICIS registration year (1 September to 31 August).
Example - using equation 1
A company is introducing 2,000 kg of a chemical for end use in commercial paint and 3,500 kg of the chemical for end use in paint primers.
The ECV will be: 2,000 + 3,500 = 5,500 kg
Multiply the total volume of your chemical that you will introduce in an AICIS registration year (1 September to 31 August) by the RRF value.
ECV = IV x RRF
IV = Introduction volume. This means the total importation and manufacture quantity of your chemical in kg in an AICIS registration year (1 September to 31 August).
RRF = The release reduction factor value that applies to your end use scenario (see table of end use scenarios and their RRF values).
Example - using equation 2
A company is introducing 6,000 kg of a chemical for end use in plastic products in Australia. The RRF for this end use is 0.05.
The ECV will be: 6,000 × 0.05 = 300 kg
Equation 2: detailed method (single end use; with RRF)
Option 1: Use our online tool
Go to our environment categorisation volume calculator
Option 2: Calculate manually using the equation
- Look at the release reduction factor (RRF) table below and identify which end use scenarios apply to your introduction.
- Select one of the end use scenarios and calculate the Environment categorisation volume using the instructions for equation 4.
- Repeat B) for all of your end uses and then add them up to get your total Environment categorisation volume using the equation below.
ECV = (IV1 x RRF1) + (IV2 x RRF2) +… + (IVn x RRFn)
Note: IVn = the introduction volume for end use ‘n’
Example of using equation 5
In this scenario, the company knows the breakdown of the volumes for each end use:
- 6,000 kg manufactured in Australia that will be exported for end use overseas (RRF for this end use is 0.05).
- 5,000 kg for end use in paints within Australia (RRF for this end use is 0.05).
- 1,000 kg for end use in fabric products in Australia (RRF for this end use is 0.4).
The introduction volume is multiplied by the RRF for each end use scenario before adding these together to get the total ECV.
The ECV will be: (6,000 × 0.05) + (5,000 × 0.05) + (1,000 × 0.4) = 1,050 kg;
If your introduction has multiple end uses
Select one of the options:
- Use Equation 3
- Use Equation 4
- Use Equation 5
You need to use equation 3 or 4 if you don’t know the total introduction volume for every end use for your chemical.
Equation 3: simplest method (multiple end uses; no RRF)
ECV = IV
IV = Introduction volume. This means the total importation and manufacture quantity of your chemical in kilograms (kg) in an AICIS registration year (1 September to 31 August).
Equation 4: intermediate method (multiple end uses; allocate the total introduction volume to the end use that has the highest RRF)
- Look at the release reduction factor (RRF) table and identify which end use scenarios apply to your introduction.
- Find the end use scenario that has the highest RRF value. Note: the highest RRF value represents the end use with the highest level of environment exposure.
- Multiply the total volume of your chemical that you will introduce for that end use scenario in an AICIS registration year (1 September to 31 August) by the RRF value you found in (B). Do not just use the volume for one of the end uses.
ECV = IV x RRF
IV = Introduction volume. This means the total importation and manufacture quantity in kilograms (kg) of your chemical.
RRF = The highest release reduction factor value that applies to one of your end use scenarios (see table of end use scenarios and their RRF values).
Example of using equation 4
A company is introducing 12,000 kg of a chemical for multiple end uses. They do not know the volume for each end use:
- some chemical will be manufactured in Australia and exported for end use overseas (RRF for this end use is 0.05).
- some chemical will be for end use in paints within Australia (RRF for this end use is 0.05).
- some chemical will be for end use in fabric products in Australia (RRF for this end use is 0.4).
With this scenario using equation 42a the highest RRF is 10.4.
The ECV will be: 12,000 × 0.4 = 4,800 kg
Equation 5: detailed method (multiple end uses; multiple RRFs, calculate the ECV for each end use and add together)
Use equation 5 if:
- you know the annual introduction volume of your chemical for each end use
- you are willing to keep track of any changes to your introduction volume for each end use - this helps you make sure that the indicative environmental risk of your introduction does not increase.
Option 1: Use our online calculator
Go to our environment categorisation volume calculator
Option 2: Calculate manually using the equation
Instructions – calculate manually
- Look at the release reduction factor (RRF) table and identify which end use scenarios apply to your introduction.
- Select one of the end use scenarios and calculate the Environment categorisation volume using the instructions for equation 4.
- Repeat B) for all of your end uses and then add them up to get your total Environment categorisation volume using the equation below.
ECV = (IV1 x RRF1) + (IV2 x RRF2) +… + (IVn x RRFn)
Note: IVn = the introduction volume for end use ‘n’
Example of using equation 5
In this scenario, the company knows the breakdown of the volumes for each end use:
- 6,000 kg manufactured in Australia that will be exported for end use overseas (RRF for this end use is 0.05).
- 5,000 kg for end use in paints within Australia (RRF for this end use is 0.05).
- 1,000 kg for end use in fabric products in Australia (RRF for this end use is 0.4).
The introduction volume is multiplied by the RRF for each end use scenario before adding these together to get the total ECV.
The ECV will be: (6,000 × 0.05) + (5,000 × 0.05) + (1,000 × 0.4) = 1,050 kg
Release reduction factors (RRFs) for different end use scenarios
The RRF values range between 0 and 1. A low release reduction factor indicates that only a small portion of the introduction volume is likely to contribute to environmental exposure. A higher release reduction factor indicates that a higher proportion of the introduction volume could contribute to environmental exposure.
| Your introduction's end use scenario | RRF value |
|---|---|
| Chemical imported into Australia; import containers remain closed; then exported for end use overseas | 0 |
| Chemical imported into Australia; limited handling of the chemical (such that import containers are opened); then exported for end use overseas | 0.05 |
| Chemical manufactured in Australia; exported for end use overseas | 0.05 |
| Adhesive and sealant products (end use in Australia) | 0.05 |
| Apparel and footwear care products (end use in Australia) | 0.05 |
| Arts, crafts and hobby products (end use in Australia) | 0.05 |
| Explosive products (end use in Australia) | 0.05 |
| Fuel, oil, fuel oil additives and related products (end use in Australia) | 0.05 |
| Lubricant and grease products (end use in Australia) | 0.05 |
| Personal care products - limited environmental release (end use in Australia) | 0.05 |
| Tattoo ink products (end use in Australia) | 0.05 |
| Paint and coating products (end use in Australia) | 0.05 |
| Plastic and polymer products (end use in Australia) | 0.05 |
| Construction products not covered by other end uses (end use in Australia) | 0.2 |
| Fabric, textile and leather products not covered by other end uses (end use in Australia) | 0.4 |
| Electronic products (end use in Australia) | 0.5 |
| Ink, toner and colourant products (end use in Australia) | 0.8 |
| Air care products (end use in Australia) | 1 |
| Anti-freeze and de-icing products (end use in Australia) | 1 |
| Automotive care products (end use in Australia) | 1 |
| Cleaning and furniture care products (end use in Australia) | 1 |
| Laundry and dishwashing products (end use in Australia) | 1 |
| Extractive products not covered by other end uses (end use in Australia) | 1 |
| Paper products (end use in Australia) | 1 |
| Personal care products not covered by other end use (end use in Australia) | 1 |
| Photographic products (end use in Australia) | 1 |
| Water treatment products (end use in Australia) | 1 |
| Any other end use not covered above (end use in Australia) | 1 |
Product definitions and examples from the RRF table
Adhesive and sealant products means an end use to fasten other materials together or stop the passage of liquid or gas. Examples include:
- glues
- binders
- adhesives
- pastes
- sealants
- fillers
- putties
- solder and caulking compounds
- spray foam insulation
Apparel and footwear care products means an end use to care for apparel and footwear products intended for consumer and commercial use. Examples include:
- footwear polishes
- waxes and stains to waterproof and improve appearance and other desirable properties
- apparel surface treatment products for water, stain or flame resistance
Arts, crafts and hobby products means an end use in arts, crafts or hobbies. Examples include:
- crafting paints
- crafting glue
- adhesives (e.g. solder and hot-melt adhesives)
- fixatives
- finishing spray coatings and modelling clay
Explosive products means an end use for producing a sudden expansion, usually accompanied by production of heat and large changes in pressure. Examples include:
- pyrotechnics
- high explosives and propellants
- igniters
- primers
- initiatory
- illuminants
- smoke and decoy flares
- incendiaries
Fuel, oil, fuel oil additives and related products means an end use as:
- liquid fuel in containers used for cooking, heating or for power in vehicles or appliances, or
- a fuel additive to inhibit corrosion, provide lubrication, increase efficiency of use, or decrease production of undesirable by-products.
Examples of liquid fuels include:
- gasoline
- diesel fuels
- kerosene
- lamp oils
Examples of fuel oil additives include:
- stabilisers
- anti-knock agents
- corrosion inhibitors
- detergents
- fuel dyes
- oxygenates
- antioxidants
- odour agents
Lubricant and grease products means an end use in a liquid, paste or spray to reduce friction, heat generation and wear between solid surfaces. Examples include:
- engine oils
- transmission, brake and hydraulic fluids
- gear oils
- calcium, sodium, lithium, and silicone-based greases
Personal care products – limited environmental release means an end use in solid or hardening personal care products (including cosmetics) that are primarily disposed of to landfill. Examples include:
- baby wipes
- facial tissues
- nail care products including nail polish and remover
Tattoo ink products means an end use in a combination of industrial chemicals that contains one or more colouring agents and is applied to the dermal layer of the skin for the purposes of colouring the skin. Examples include:
- pigments
- dyes
- resins
Paint and coating products means an end use to paint or coat substrates intended for consumer or commercial use. Examples include:
- decorative coatings
- automotive coatings
- transportation coatings
- wood finishes
- powder coatings
- coil coatings
- packaging finishes
- general industrial coatings
- automotive refinish
- industrial maintenance and protective coatings
- marine coatings
- thinners
- removers
Plastic and polymer products means an end use in production of plastics or polymers. Examples include:
- monomers
- initiators
- additives
Construction products not covered by other end uses means an end use in construction materials, except where another scenario covers the end use. Examples include:
- additives in cements and dry mortar
- additives to bitumen for road repair
- internal release agents for thermo-set laminating resins
- resins in particle board manufacture
- wood substitutes used to make mouldings
- resins used in the production of composite materials
Fabric, textile and leather products not covered by other end uses means an end use to impart colour and other desirable properties onto fabric, textiles, and leather products that are intended for consumer or commercial use.
These properties include:
- water/soil/stain repellence
- wrinkle resistance
- flame resistance
Examples of this type of product include:
- textile dyes
- textile finishing agents
- leather tanning products
- leather dyes
- leather finishing agents, leather conditioner and surface treatment products
Electronic products means an end use in the production of electronic components. Examples include:
- chemicals in vapour deposition
- electroless plating
- electroplating
- etching
- high vacuum evaporation/sputtering
- laminate processing
- soldering
- photolithography
Ink, toner and colourant products means an end use for:
- writing
- printing
- creating an image on paper and other substrates
- applying to substrates to change their colour or hide images
Examples of this type of product include:
- pigmented liquid
- toners or powders used in copy machines and toner/printer cartridges
- inks used in writing equipment
- inks for stamps and correction fluids and tapes
This category does not include pigments and colourants added to paints and coatings.
Air care products means an end use to odorise or deodorise indoor air in homes, offices, motor vehicles, and enclosed spaces and intended for consumer or commercial use. Examples include:
- aerosol sprays
- liquid/solid/gel diffusers
- air fresheners
- scented candles
- incense
Anti-freeze and de-icing products means an end use:
- as an additive to fluids, especially water, to reduce the freezing point of the mixture, or
- applied to surfaces to melt or prevent build-up of ice
Examples of this type of product include:
- anti-freeze liquids
- de-icing liquids (windshield de-icers, aircraft de-icers)
- de-icing solids (ice melting crystals)
- lock de-icers
Automotive care products means an end use (intended for consumer or commercial use) to clean and care for exterior and interior surfaces of automotive vehicles. Examples include:
- car waxes
- polishes
- waterproofing products for windshield or automotive window glass
- cleaners
- sealers
- car wash solutions
- vinyl/rubber/plastic protectants
- automotive carpet and upholstery cleaners
- wheel and tyre care products
- exterior trim protectants
- touch-up paint products
Cleaning and furniture care products means an end use (intended for consumer or commercial use) to:
- remove dirt, grease, stains, and foreign matter from furniture and furnishings
- cleanse, sanitise, bleach, scour, polish, protect, or improve the appearance of surfaces
Examples include:
- cleaners used on glass, floors, tub and tile, ovens and drains
- scouring powders
- dusting products
- waxes
- polishes
- stain repellent sprays
Laundry and dishwashing products means an end use in liquid, granular, gel or unit dose packets/tablets to:
- remove food residue from dishes
- remove dirt from textiles
- enhance properties of textiles
- remove stains from textiles
Examples include:
- dishwashing detergents and laundry detergents
- stain removers and fabric enhancers
- bleach
- rinse aids
- lime and rust removers
- dry cleaning products used in non-aqueous cleaning processes
Extractive products not covered by other end uses means an end use in:
- mining
- onshore drilling
- related activities such as extraction, cementing, hydraulic fracturing, refining
These scenarios do not include end use in offshore drilling. This end use is a designated kind of release into the environment (for which you do not calculate an ECV).
Paper products means an end use in paper production. Examples include:
- effluent treatment chemicals
- maintenance chemicals
- deposit and cleaning agents
- defoamers
- surfactants
- polymeric retention aids
- coagulants
- clay
- resins
Personal care products not covered by other end uses means an end use for cosmetic use, except those covered under the “personal care products - limited environmental release end use” scenario. Examples include:
- bath and shower products
- make-up products
- hair, oral and skin care products
- secondary sunscreen products
- deodorants
- perfumes
Photographic products means an end use (for consumer or commercial use) to take photographic images, develop and process film, and make photographic prints. Examples include:
- processing solutions (for developing, stopping, and fixing photos)
- chemicals used in the manufacture or processing of film or photographic paper
Water treatment products means an end use to treat water in cooling and heating systems (including industrial heat-exchanger systems) and potable water supplies. Examples include:
- chemicals used in pH buffers
- scale and corrosion inhibitors
- flocculating agents
- ion exchange resins
This scenario does not include end uses to treat municipal water supplies or other large-scale water supplies for human or animal consumptions or irrigation. These end uses involve a designated kind of release into the environment (for which you do not calculate an environment categorisation volume).
Appendix: Calculate your human health categorisation volume
You need to work out your human health categorisation volume (HHCV) when you are working out which human health exposure applies to your introduction at step 4.3.
If your introduction has a single end use
Use either equation 1 or 2.
Equation 1: simplest method (single end use; no exposure reduction factor (ERF))
HHCV = IV
IV = Introduction volume. This means the total importation and manufacture quantity of your chemical in kilograms (kg) in an AICIS registration year (1 September to 31 August).
Example - using equation 1
A company is introducing 1,000 kg of a chemical manufactured in Australia that will be exported for end use overseas and 500 kg of chemicals that are in skin care products.
The HHCV will be: 1,000 + 500 = 1,500 kg
Equation 2: detailed method (single end use; with ERF)
Option 1: Use our online tool
Go to the human health categorisation volume calculator
Option 2: Calculate manually using the equation
A) Look at the exposure reduction factor (ERF) table and identify which end use scenario applies to your introduction and the corresponding ERF value.
B) Multiply the total volume of your chemical that you will introduce in an AICIS registration year (1 September to 31 August) by the ERF value.
HHCV = IV x ERF
IV = Introduction volume. This means the total importation and manufacture quantity of your chemical (in kg) in an AICIS registration year (1 September to 31 August).
ERF = The exposure reduction factor value that applies to your end use scenario (see table of end use scenarios and their ERF values).
This page accompanies step 4.3 of the Guide to Categorising your chemical importation and manufacture.
Example - using equation 2
A company is introducing 6,000 kilograms of a chemical manufactured in Australia that will be exported for end use overseas. The ERF for this end use is 0.05.
The HHCV will be: 6,000 × 0.05 = 300 kilograms
If your introduction has multiple end uses
Select one of the options:
- Use Equation 3: simplest method
- Use Equation 4: intermediate method
- Use Equation 5: detailed method
You need to use equation 3 or 4 if you don't know the total introduction volume for every end use for your chemical.
Equation 3: simplest method (multiple end uses; no exposure reduction factor)
HHCV = IV
IV = Introduction volume. This means the total importation and manufacture quantity of your chemical in kilograms (kg) in an AICIS registration year (1 September to 31 August).
Equation 4: intermediate method (multiple end uses; allocate the total introduction volume to the end use that has the highest ERF)
Instructions
A) Look at the exposure reduction factor (ERF) table and identify which end use scenarios apply to your introduction.
B) Find the end use scenario that has the highest ERF value. Note: the highest ERF value represents the end use with the highest level of human exposure.
C) Multiply the total volume of your chemical that you will introduce for that end use scenario in an AICIS registration year (1 September to 31 August) by the ERF value you found in (B). Do not just use the volume for one of the end uses.
HHCV = IV x ERF
IV = Introduction volume. This means the total importation and manufacture quantity in kilograms (kg) of your chemical. ERF = The highest exposure reduction factor value that applies to one of your end use scenarios (see table of end use scenarios and their ERF values).
Example - using equation 4
A company is introducing 12,000 kg of a chemical for multiple end uses. They do not know the volume for each end use:
- some chemical will be manufactured in Australia and exported for end use overseas (ERF for this end use is 0.05).
- some chemical will be for end use in paints within Australia (ERF for this end use is 0.1).
- some chemical will be for end use in specified consumer products in Australia (ERF for this end use is 1).
With this scenario using equation 2a the highest ERF is 1.
The HHCV will be: 12,000 × 1 = 12,000 kg
Equation 5: detailed method (multiple end uses; multiple ERFs, calculate the HHCV for each end use and add together)
Use equation 5 if:
- you know the annual introduction volume of your chemical for each end use
- you are willing to keep track of any changes to your introduction volume for each end use - this helps you make sure that the indicative human health risk of your introduction does not increase.
Option 1: Use our online tool
Go to our human health categorisation volume calculator
Option 2: Calculate manually using the equation
A) Look at the exposure reduction factor (ERF) table and identify which end use scenarios apply to your introduction.
B) Select one of the end use scenarios and calculate the human health categorisation volume using the instructions for equation 4.
C) Repeat B) for all of your end uses and then add them up to get your total human health categorisation volume using the equation below.
HHCV = (IV1 x ERF1) + (IV2 x ERF2) +… + (IVn x ERFn)
Note: IVn = the introduction volume for end use ‘n’
Example - using equation 5
In this scenario, the company knows the breakdown of the volumes for each end use:
- 6,000 kg manufactured in Australia that will be exported for end use overseas (ERF for this end use is 0.05).
- 5,000 kg for end use in paints within Australia (ERF for this end use is 0.1).
- 1,000 kg for end use in specified consumer products in Australia (ERF for this end use is 1).
The introduction volume is multiplied by the ERF for each end use scenario before adding these together to get the total HHCV.
The HHCV will be: (6,000 × 0.05) + (5,000 × 0.1) + (1,000 × 1) = 1,800 kg
Exposure reduction factors (ERFs) for different end use scenarios
The ERF values range between 0 and 1. A low exposure reduction factor indicates that only a small portion of the introduction volume is likely to contribute to human exposure. A higher exposure reduction factor indicates that a higher proportion of the introduction volume could contribute to human exposure.
| Your introduction's end use scenario | ERF value |
|---|---|
| Chemical imported into Australia; import containers remain closed; then exported for end use overseas | 0 |
| Chemical imported into Australia; limited handling of the chemical (such that import containers are opened); then exported for end use overseas | 0.05 |
| Chemical manufactured in Australia; exported for end use overseas | 0.05 |
| Specified consumer products with end use in Australia* | 1 |
| All other end uses in Australia | 0.1 |
Note *Specified consumer products means any of the following products:
- cosmetics
- nasal sprays
- ear sprays
- intimate lubricants
- massage oils and gels
- products applied to the nails to harden, or deter the biting of, nails
Specified consumer products do not include tattoo inks. If your chemical has an end use in tattoo inks, its introduction is automatically in human health exposure band 4 and you do not need to calculate a HHCV.